The West Lab
The West Lab investigates how mitochondria shape immune and inflammatory responses in genetic disorders and aging-related diseases.
Mitochondria are multifaceted organelles integral to many cellular processes including energy generation and programmed cell death. More recently, mitochondria have emerged as central hubs in the mammalian immune system, orchestrating signal transduction cascades and metabolic switches necessary for the activation and survival of immune cells. Mitochondria are also important sources of damage-associated molecular patterns (DAMPs), which can trigger inflammatory responses when released from the organelle. Given their pleiotropic roles, mitochondrial dysfunction is increasingly implicated as a cause or accelerant of numerous human diseases.
Research in the West Lab aims to: 1) define how mitochondria function as central regulators of innate immune and inflammatory responses, and 2) delineate how the mitochondrial-innate immune axis contributes to disease pathobiology. We pursue a multidisciplinary approach by integrating immunology, cell biology, and physiology using engineered mouse models of disease, multi-omics, flow cytometry, and super-resolution imaging.
Scientific Report
Mitochondrial DNA Sensing by the cGAS-STING Pathway in Immunity and Disease
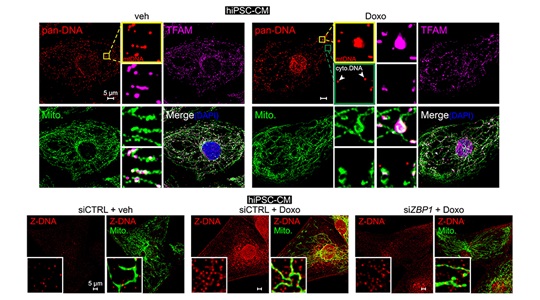
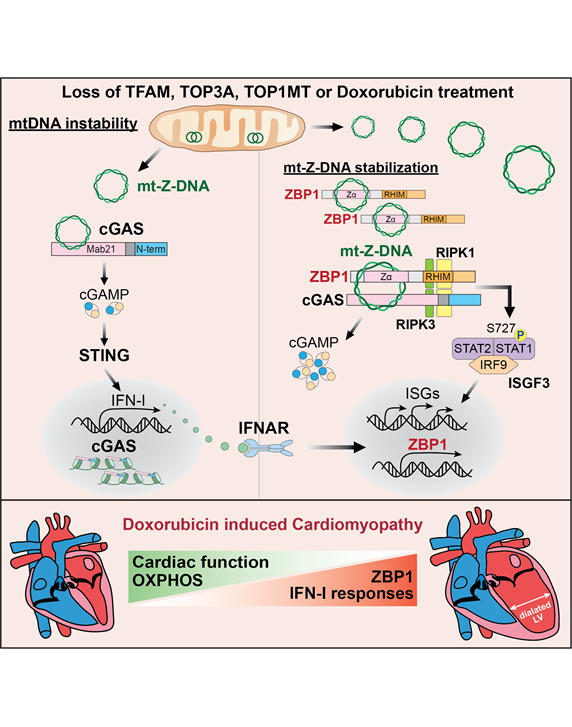
Mitochondrial DNA (mtDNA) is a small, maternally inherited, circular DNA genome housed in the matrix of mitochondria. My lab is exploring the mechanisms by which mitochondrial dysfunction leads to mtDNA release and activation of the cyclic GMP-AMP synthase (cGAS) – Stimulator of Interferon Genes (STING) innate immune pathway (see comprehensive reviews: PMID: 28393922; PMID: 28765055). cGAS is a sensor of pathogen-derived and endogenous DNA that signals via STING to trigger the expression of type I interferons (IFN-I) other cytokines. These cytokines promote pathogen clearance but may also lead to damaging inflammation if unconstrained. Our ongoing research in this area aims to define how the cGAS-STING pathway is regulated and characterize how it senses cytosolic mtDNA in genetic disorders and aging-related diseases. To this end, my group has developed comprehensive and readily adaptable methods to assess mtDNA release and cGAS activation in mammalian cells (PMID: 35175686). Most recently, we have uncovered that cGAS forms a novel complex with the DNA sensor ZBP1 to detect a unique Z-form of mtDNA released during mitochondrial genome stress. In addition, we have found that the cGAS-ZBP1 complex sustains IFN-I signaling in response to mtDNA instability and release, which promotes cardiomyocyte injury and heart failure in chemotherapy-related cardiotoxicity (PMID: 37352855).
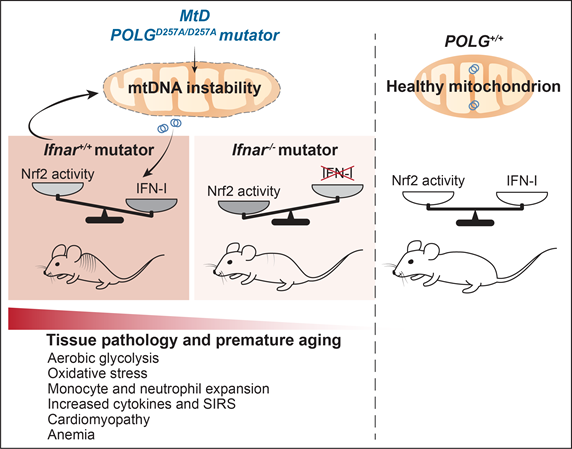
Inborn Errors of Innate Immunity in Inherited Mitochondrial Disorders
Mitochondrial diseases (MtD) are a group of clinically heterogeneous disorders caused by inherited mutations in genes that function in mitochondrial metabolism. In addition to exhibiting metabolic and energetic deficits, patients with MtD are more susceptible to opportunistic pathogens and suffer elevated complications arising from these infections. Although B and T cell immunodeficiency can contribute to recurrent infections in MtD, comparatively little is known about innate immune dysregulation in patients or animal models. My lab is utilizing novel mouse models that faithfully recapitulate aspects of human MtD to characterize innate immune responses at rest and during infection. For example, we recently showed that the polymerase gamma (PolG) mutator model of mitochondrial DNA instability exhibits a hyperinflammatory innate immune status that is driven by monocyte expansion and heightened STING-dependent IFN-I responses. We discovered that persistent IFN-I signaling represses nuclear factor erythroid 2-related factor 2 (Nrf2) activity, which increases oxidative stress and aerobic glycolysis that potentiate inflammation and multi-organ pathology (PMID: 34039599). We have also reported mtDNA instability and heightened IFN-I responses in CLPP-null mice, a faithful model of the rare mitochondrial disorder Perrault Syndrome, (PMID: 33731338, PMID: 34345994). In collaboration with Dr. Peter McGuire’s group at the NIH/NHGRI, we have uncovered immune and inflammatory signatures in pediatric patients with MtD, paralleling our work in mouse models (PMID: 37208779). Our ongoing studies are focused on unraveling how innate immune hyperactivity and myeloid cell dysfunction in models of MtD predispose to opportunistic respiratory pathogens, lung inflammation, and metabolic decompensation.
Mitochondrial Dysfunction in Cancer and Toxicant-Related Inflammation
Over the past several years, our team has worked collaboratively to define how mtDNA stress increases pro-tumorigenic innate immune signatures in melanoma (PMID: 30242167, PMID: 32395698). We also are actively collaborating with Dr. Robert Dantzer’s group at MD Anderson Cancer center to characterize how mitochondrial dysfunction and inflammation contribute to behavioral fatigue resulting from cancer and cancer therapy (PMID: 34418499, PMID: 37076053). Finally, our lab has uncovered that persistent mitochondrial dysfunction appears to be at the core of Gulf War Illness (GWI), a chronic, multi-symptom inflammatory disorder affecting nearly 30% of Gulf War veterans. We have found that mitochondrial stress and downstream activation of cGAS-STING and NLRP3 contribute to disease progression in a toxicant-induced model of GWI (PMID: 34333111). We are currently exploring whether targeted ablation/inhibition of these innate immune pathways could be an effective approach to alleviate neuroinflammation and cognitive deficits frequently observed in veterans with GWI.