Cytokine release syndrome poses a unique and complex challenge to the development and administration of antibody-based therapeutics such as bispecific T cell engagers and monoclonal antibodies. JAX Cytokine Release Syndrome (CRS) Evaluation Studies are built on humanized platforms to provide the most translationally relevant data to assess antitumor antibodies which may induce CRS and immunosuppressive antibodies for the prevention or resolution of CRS.
Using the JAX in vivo CRS Evaluation Study provides data on the systemic consequences of high cytokine release which cannot be achieved using in vitro assays and avoids the high cost and limited availability of non-human primates.
JAX CRS Evaluation Studies provide predictive data regarding your therapeutic’s properties, allowing you to identify the most promising lead candidate and fine-tune the dosing to maximize the likelihood of clinical success.
PBMC-humanized mice were treated with CD19xCD3 bispecific, OKT3, or anti-CD28, and human cytokine levels were measured 6 hours post-treatment. The Venn diagram illustrates human cytokines that were significantly induced by these therapeutics compared to the PBS control. Some cytokines were induced in a therapy-specific manner, while others were commonly induced by all three therapeutics. Notably, the PBMC-humanized mouse platform was able to detect these differences in therapeutic responses.
Compare different lead molecules showing functional activity using a single PBMC donor for the study.
Compare lead candidates to current standard of care therapies and determine which molecule / dosage provides minimal toxicity by using pre-characterized PBMC donors.
Test the optimal dosages against the diversity of the population by using multiple PBMC donors in the study.
JAX’s humanized platform for the evaluation of antibody safety and efficacy assesses therapeutics in the context of the human immune system as they engage human tumors or other tissues. This platform provides a much more scalable, reproducible, robust and translationally relevant examination of immunotherapeutic activity than common models such as in vitro assays or non-human primate models, which can fail to mimic the human condition or account for simultaneous engagement of epitopes on tumors and immune cell targets for engagement.
JAX's humanized CRS platform enables researchers to evaluate crucial aspects of the safety of bispecific and multispecific antibodies, including their cytokine responses. Since multispecific antibodies usually elicit their activity by binding to antigens on both cancerous and immune cells, it is essential to study the immune system response to this class of therapeutic molecules in the presence of both targets. Furthermore, individual patients may respond differently to the same treatment due to multiple factors, including inherent differences in their immune system. JAX's humanized platforms allow the engraftment of human tumors and the inclusion of multiple PBMC donors. This enables the assessment of donor-to-donor variability in the presence of target tumor cells, providing a more realistic prediction of clinical outcomes.
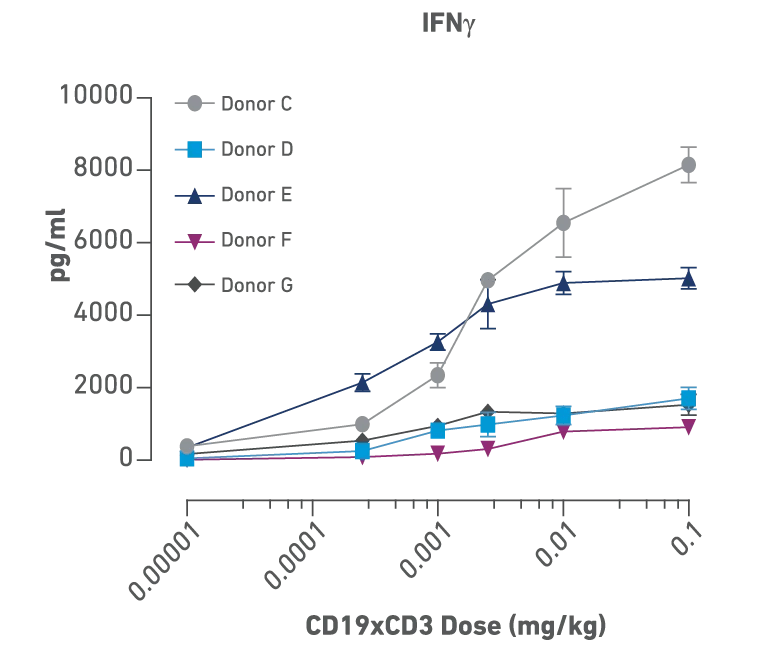
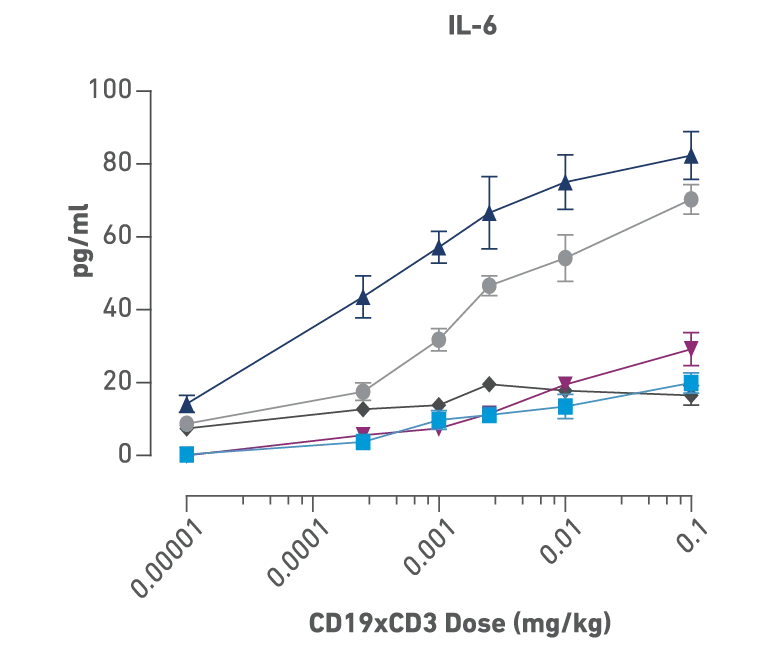
Figures above:
Identification of donor- and dose-dependent cytokine release and efficacy with CD19xCD3 bispecific treatment in PBMC humanized NSG-MHC I/II-DKO mice bearing human B cell lymphoma. Mice were humanized with 5 different human PBMC donors followed by engraftment with a luciferase tagged Raji B cell lymphoma. Mice were then dosed with PBS or six different concentrations of CD19xCD3 bispecific. The cytokine levels show dose dependency, and each PBMC donor showed a unique profile of cytokine release.
Figure above:
Donors D, F, G: these patients had a good response even with low cytokine release, so they could have tolerated an even higher dose and perhaps seen even further improved efficacy, Donor C: high cytokine release and poor overall response.
JAX’s humanized platforms allow you to evaluate therapeutic articles, testing multiple candidates, doses, tumor types, and timepoints simultaneously more efficiently and predictively. Work with our experts to select the right mouse strains, donors, tumor models, measures, and endpoints to get a complete picture of your therapeutic’s safety profile. Choose your preferred tumor cell line(s), or test against reference compounds in the absence of tumors. Then let us guide you in selecting PBMC donors, mouse models, and a battery of informative assays.
| Study Design | Detail |
|---|---|
| Models | NSG™ and Variants such as NSG-MHC Class I/II DKO Adult PBMC from single (for lead selection) or multiple donors (for population assessment) |
| Groups | Minimum 3 arms with 5 mice / arm, 6 - 16 days* *Long term safety studies can be performed as well. Study design and duration would vary depending on the client's goal, test article and type of tumor involved |
| Data |
|
In vivo CRS assays can help reduce risks and speed up the process of delivering effective therapies to patients. By using these assays, drug developers not only can evaluate the risk-benefit ratio of preclinical candidates but also identify the most promising lead compounds early in the development pipeline, so they can allocate resources more effectively and advance to clinical stages more quickly and confidently.
Not what you're looking for?
Visit our Resource Hub