There is no doubt the blockbuster hit Avatar is science fiction. 3D patient avatars for cancer, on the other hand, may soon become a reality. These 3-dimensional representations of unique tumors, as described by Jeff Chuang and peers, may be the next big thing for precision medicine.
Glance in the rearview mirror
Cancer modeling research has come a long way. Before jetting off to the future, it is important to recognize how upcoming models came to be, beginning with a tried-and-true method, cell culture. Developed in the early 1900s, cancer cell culture involves collecting and isolating cancerous cells and nourishing them in a sterile environment to stimulate growth. The cancer cells grow in a thin 2-dimensional layer (“2D cell culture”) that can be perpetuated continually if desired, allowing researchers to investigate cellular characteristics and changes that may contribute to disease.
Simple and cost effective, 2D cell cultures, and the cell lines produced from them, have been incredibly important resources for cancer research. From understanding cell biology to explaining disease onset to predicting drug outcomes, much scientific discovery has been attributed to studies using a 2D layer of cells. While cancer biology research and clinical testing will continue to utilize such 2D cell culture models, there are now new ways of more accurately recreating both the complexity of the cells within a tumor as well as the diverse set of cells that interact with the cancer during tumor progression.
Hitting the pavement
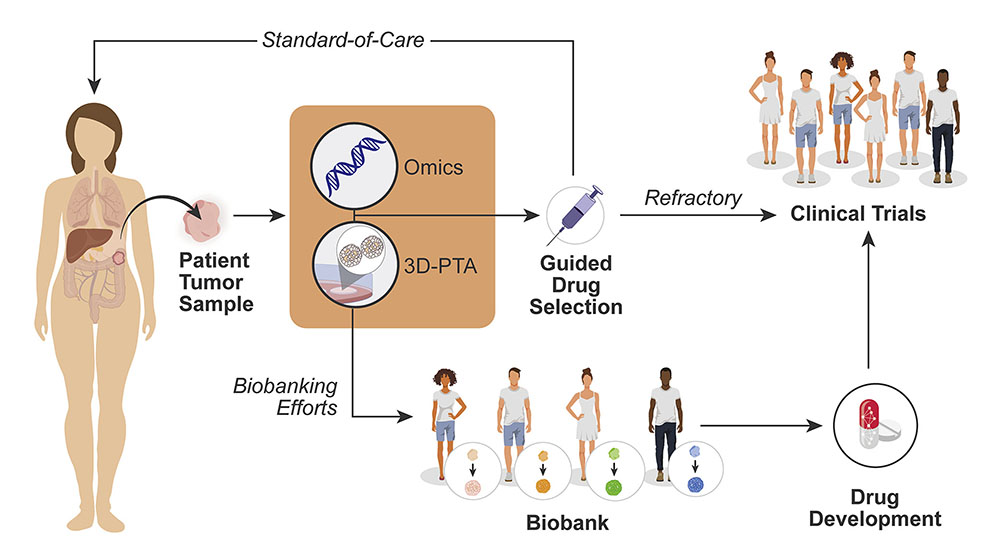
3D cell culturing methods have been widely adopted over the last few years to overcome the limitations of conventional 2D methods. Jeffrey Chuang, Ph.D., a professor at The Jackson Laboratory for Genomic Medicine and Deputy Director of The Jackson Laboratory (JAX) Cancer Center, has summarized the power of 3D models and mapped out the key milestones required for implementation of these powerful tools. In “A path to translation: How 3D patient tumor avatars enable next generation precision oncology,” published in Cancer Cell, Chuang and colleagues, including fellow JAX professors Paul Robson, Ph.D., and Martin Pera, Ph.D., present how to bring the newest form of patient-cancer modeling (i.e., 3D patient tumor avatars or 3D-PTAs), from the bench to the clinic.
2D cultures have important limitations. For example, they usually contain only the fastest growing cells on the plate and fail to capture the cellular diversity found in and around tumors. To recapitulate more of the organization and complexity of cells within and around a tumor, researchers developed patient-derived xenograft (PDX) models, created using tumor cells or tissue from a patient and implanting them into an immunocompromised mouse. Chuang’s research has contributed to the characterization of hundreds of PDX cancer models produced at JAX, including tumors of the breast, lung, bladder, and more. These human patient models capture the genetic diversity and physical aspects of each unique tumor in an in vivo environment.
While PDX models are a key system for testing drugs before starting clinical trials, they take a few months to be established from a patient, and then treatment studies take another several months. The time-intensive process makes it difficult to use PDX mice as patient-specific avatars. Therefore, while Chuang and colleagues agree that PDX models will remain a key resource for cancer biology research, the potentially more efficient 3D culture models are promising systems to develop in precision oncology to improve therapeutic decisions.
Removing roadblocks
Among the exciting 3D-PTAs currently being refined are patient-derived organoids (PDOs). Like PDX models, PDOs are cultured from a patient’s own unique tumor cells. But while PDX models are grown in mice, PDO models grow in a 3D matrix that simulates the 3D environment within an organ. Only taking a few weeks to develop, PDOs are quicker to establish and have proven to be effective systems for predicting drug response. Other kinds of 3D-PTAs have the potential to be scaled down to fit on a microchip, unlocking the ability to produce accurate patient models faster than ever before. With the combination of genetic sequencing, protein studies, and more, there has never been a more up close and personal view of cancer at scientists’ fingertips.
What is the next step for implementing 3D-PTAs as patient avatars in the clinic? Standardizing them remains a top priority. Findings must be repeatable, precise and accurate between all researchers and clinicians, and 3D-PTAs are no exception. The extracellular matrix scaffolds, culturing media and growth factor combinations that are best for each 3D-PTA and each tumor type need to be established and consistently implemented. Standardized patient data collection is also vital to expand the capabilities of 3D-PTA libraries. Finally, clinical trials assessing the potential of 3D-PTAs need to be undertaken. The new models themselves may also contribute to modernizing clinical trials, leading to effective implementation of genome testing-guided, patient-stratified trials.
While there are still some significant challenges to address and bridges to cross, Chuang and colleagues conclude that 3D-PTAs have the potential to change the landscape of precision medicine. If moved with proper rigor and thoughtfulness into the clinic, they represent a clinical resource that can inform truly personalized cancer care.