Investigating TDP-43 and its regulation of stathmin-2
Research Highlight | March 16, 2023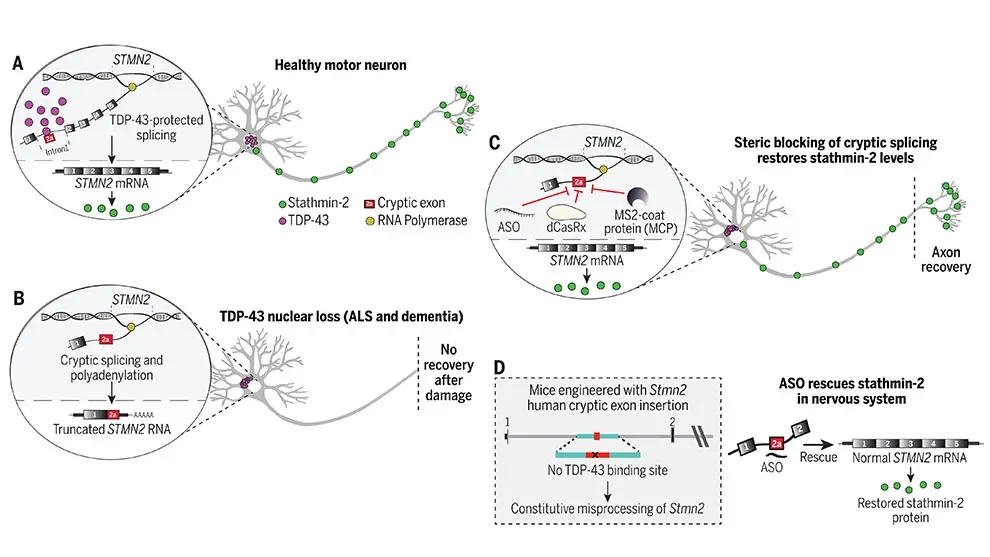
A group of neurological disorders, including amyotrophic lateral sclerosis (ALS), Alzheimer’s disease and frontotemporal dementia, are characterized by the mislocalization of TDP-43 outside the nucleus and aggregation in the cellular cytoplasm.
Scientists have found that TDP-43 is essential for proper maturation of the pre-mRNA encoding stathmin-2, a protein necessary for the outgrowth of neurons’ axons during development and regeneration following any injury. In a paper in Science, researchers led by a group at the University of California, San Diego, and including Cathleen Lutz, Ph.D., of The Jackson Laboratory, determined how TDP-43 regulates pre-mRNA processing and proper production of stathmin-2. In addition, they identified rescue antisense oligonucleotides that restore stathmin-2 production and axonal regeneration capacity in neurons lacking TDP-43.
The restoration of axonal regeneration was observed in both cultured human motor neurons as well as mice with genes edited to contain the human sequences associated with aberrant splicing. The findings point to a promising potential therapeutic approach for ALS and other TDP-43-associated diseases and conditions.
The University of California, San Diego, prepared an article that provides more details about the paper and its implications.
There is an accompanying perspective on the paper in the same issue of Science.

