Researchers in Australia and North America have recently identified a novel parvovirus in laboratory mice: the Mouse Kidney Parvovirus (MKPV; Roediger B et al., 2018). Though it has only recently been identified, this virus is suspected to be responsible for a chronic progressive kidney disease in immunodeficient mice that has been described for over 40 years.
Mouse Kidney Parvovirus: Here’s what you need to know
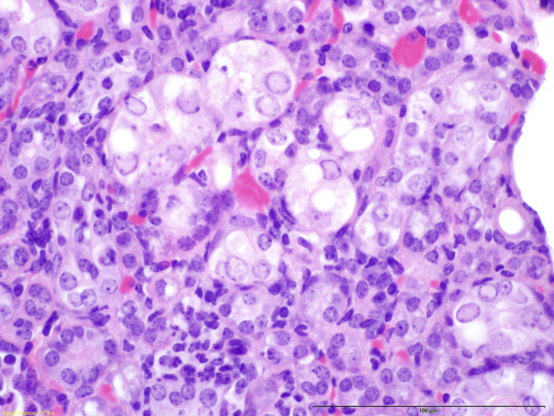
Blog Post | August 22, 2019Researchers in Australia and North America have recently identified a novel parvovirus in laboratory mice: the Mouse Kidney Parvovirus (MKPV; Roediger B et al., 2018). A similar virus was previously identified in wild mice and classified as Murine Chappaparvovirus, suggesting that these two viruses are the same, and belong to a genus that is divergent from other parvoviruses of mice (Williams, 2018; Penzes, 2019; Souza 2017). This pathogen has also been labeled as “inclusion body nephritis/nephropathy” based on its histological characteristics (Barthold SW et al., 2016; see figure 1). It has the potential to cause significant clinical disease in immunodeficient mice, including tubular degeneration, interstitial fibrosis, necrosis, and eventual renal failure, leading to death within 4-5 months (Roediger B et al., 2018).
As infected mice age, some of the more severely immunocompromised strains, such as NSG™ and TRP [Rag1–/–], may develop elevated blood urea nitrogen and creatinine levels, or anemia as a result of their kidney disease. Though immunocompetent mice can also be infected and may develop histological abnormalities, they do not typically exhibit observable clinical signs (Baze WB et al., 2006). Infections in athymic nude mice are also subclinical, which may be attributed to their partially-intact immune system, as adaptive immunity appears to be an important component of the response to this pathogen (Roediger B et al., 2018).
How is MPKV transmitted?
This virus is transmitted by the fecal-oral or urinary-oral route and is detectable in the serum and urine of naïve immunocompromised mice within 50-80 days of being comingled with affected animals (Roediger B et al., 2018). Immunocompetent mice, alternatively, will develop detectable levels in feces, but not reliably in serum or urine. The lack of detection in serum or urine may be due to clearance of the pathogen by adaptive immunity before it can be transmitted to the kidney.
Vertical transmission (from parent to offspring) most likely occurs by the time of weaning, leading to a high incidence of viremia in immunocompromised mice after 100 days of age, though the elimination of the virus via embryo transfer (Roediger B et al., 2018) suggests that it is not typically transmitted in utero.
How is MKPV diagnosed?
Roediger and colleagues predict a widespread distribution of this pathogen; and since their publication was released, the prevalence of MKPV in the general laboratory mouse population has been estimated to be over 15%, based on random testing of samples collected by IDEXX Bioanalytics (IDEXX Bioanalytics). PCR assays are being developed at multiple facilities and diagnostic laboratories. Though multiple institutions are still assessing the ideal sample for testing, urine, serum, and feces have proven to be reliable for screening immunocompromised mice, with feces being the most promising for immunocompetent mice (Roediger, 2018). The virus has been detected in the soiled bedding of sentinel mice as early as 2 weeks after exposure to contaminated bedding (Hart M et al., 2019).
Additionally, detection of characteristic intranuclear inclusion bodies within histology sections of the kidney can be instrumental in the identification of this pathogen within a facility (Hart M et al., 2019). To date, no such lesions have been detected in any histology samples processed from mice taken directly from The Jackson Laboratory (JAX) facilities, including severely deficient strains, such as NSG™.
What are the best ways to remove this virus from a facility?
Removal of this pathogen from a facility would most likely rely on extensive decontamination and rederivation. As stated previously, embryo transfer has proven to be an effective means of eliminating this virus from colony animals, as is the case with other murine parvoviruses. Parvoviruses are small, hydrophilic, non-enveloped viruses, and thus are considered to be less susceptible to disinfectants than most other viruses (Shek WR et al., 2015). Because of this, an eradication plan must include consideration of disinfectant efficacy. An intermediate- to high-level disinfectant, such as dilute bleach, may help eliminate the virus from contaminated surfaces (Rutala WA et al., 2008).
Prevention relies heavily on the use of biosecurity practices, such as barrier housing with microisolator caging, PPE practices, sterilization of supplies entering the facility, rederivation of animals being introduced into the facility, testing of biological materials entering the facility, limited access to animal rooms, and observation of institutional policies such as the avoidance of contact with rodent pets or wild rodents. All of these mechanisms are used at JAX.
Summary
The overall significance of this virus has yet to be fully elucidated. Immunocompetent and athymic nude mice do not appear to be at significant risk of developing clinical signs, but more severely immunocompromised strains do have the potential to develop significant pathology. It is worth noting that parvoviruses, in general, have been shown to have immunomodulatory effects (Whary MT et al., 2015). Additionally, MKPV infection has been found to drive the loss of epithelial cells, expansion of activated macrophages, and development of myofibroblasts within the kidney (Roediger B et al., 2018). Therefore, this virus may have the ability to influence research results, particularly when the kidney is being studied.
JAX is committed to presenting the global research community with up-to-date information about the latest techniques and information to ensure the safety and health of laboratory mice. Our understanding of this novel pathogen will continue to evolve as more information is obtained.
Recommended Resources
Webinar: Generate Quality Data by Ensuring the Health and Genetic Quality of Your Mouse Colonies
Blog: MAYBE IT’S NOT YOU, MAYBE IT’S YOUR MICE!
References
Barthold SW, Griffey SM, Percy DH. 2016. Pathology of Laboratory Rodents and Rabbits. Ames (IA): John Wiley and Sons, Inc.
Baze WB, Steinbach TJ, Fleetwood, ML, Blanchard TW, Barnhart KF, McArthur MJ. 2006. Karyomegaly and intranuclear inclusions in the renal tubules of sentinel ICR mice (mus musculus). Comp. Med. 56: 435–438.
Hart M, Besch-Williford C, Crim M, Livingston M. Mouse kidney parvovirus: a newly characterized parvoviral pathogen of research mice. IDEXX BioAnalytics: https://www.idexxbioanalytics.com/hubfs/IBA_84%20MKPV%20Poster%20LINK.pdf
IDEXX Bioanalytics. Novel Mouse Kidney Parvovirus (MKPV): https://www.idexxbioanalytics.com/mkpv
Penzes JJ, Souza WM, Agbane-McKenna M, Gifford RJ. 2019. An ancient lineage of highly divergent parvoviruses infects both vertebrate and invertebrate hosts. Viruses. 11: 525
Roediger B, Lee Q, Tikoo S, Cobbin JCA, Henderson JM, Jormakka M, O'Rourke MB, Padula MP, Pinello N, Henry M, Wynne M, Santagostino SF, Brayton CF, Rasmussen L, Lisowski L, Tay SS, Harris DC, Bertram JF, Dowling JP, Bertolino P, Lai JH, Wu W, Bachovchin WW, Wong JJ, Gorrell MD, Shaban B, Holmes EC, Jolly CJ, Monette S, Weninger W. 2018. An Atypical Parvovirus Drives Chronic Tubulointerstitial Nephropathy and Kidney Fibrosis. Cell 175: 1–14.
Rutala WA, Weber DJ, Healthcare Infection Control Practices Advisory Committee (HICPAC). 2008. Guideline for disinfection and sterilization in healthcare facilities. Atlanta (GA): Centers for Disease Control and Prevention.
Shek WR, Smith AL, Pritchett-Corning KR. 2015. Microbiological quality control for laboratory rodents and lagomorphs, p 463–510. In: Fox JG, Anderson LC, Otto G, Pritchett-Corning KR, Whary MT, editors. Laboratory animal medicine. San Diego (CA): Academic Press.
Souza WM, Romeiro MF, Fumagalli MJ, Modha S, de Araujo J, Queiroz LH, Durigon EL, Figueiredo LT, Murcia PR, Gifford RJ. 2017. Chapparvoviruses occur in at least three vertebrate classes and have a broad biogeographic distribution. J. Gen. Virol. 98: 225–229.
Whary MT, Baumgarth N, Fox JG, Barthold SW. 2015. Microbiological quality control for laboratory rodents and lagomorphs, p 43–149. In: Fox JG, Anderson LC, Otto G, Pritchett-Corning KR, Whary MT, editors. Laboratory animal medicine. San Diego (CA): Academic Press.
Williams S, Che X, Garcia JA, Klena JD, Lee B, Muller D, Ulrich W, Corrigan RM, Nichol S, Jain K, Lipkin WI. 2018. Viral diversity of house mice in New York City. American Society for Microbiology. 9:2.
