Humanized mice for tumor antibody production
September 30, 2014Generation of tumor-specific human antibodies in humanized NSG mice
An exciting publication in the journal mAbs (Wege et al. 2014) describes a new method for making human B cell-derived antibodies specific for novel antigens present on human cancer cells. Tumor-specific antibodies have a great advantage over chemotherapeutic agents due to their high specificity to the target cells combined with reduced complications from off-target toxicity. The challenge in creating these antibodies is two-fold: identifying the unique antigen and generating antibodies from human B cells that recognize the tumor antigens. Wege et al. accomplished thisin vivo by co-injecting human hematopoietic stem cells and human breast cancer cells into immunodeficient NSG mice. The engrafted mice developed a human immune system, including antibodies in serum that were specific to the engrafted tumor cells.
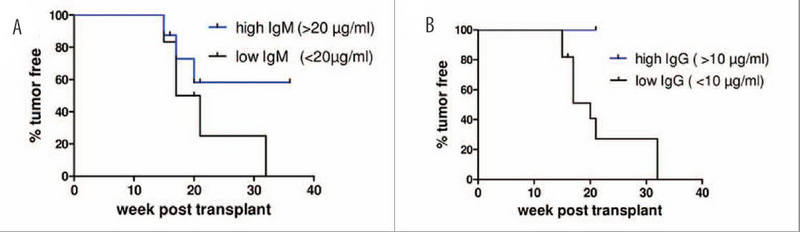
NSG mice were co-injected with human CD34+ hematopoietic stem cells and BT474 HER2+ human breast cancer cells. Human B cells produced IgM (A) and IgG (B). Higher concentrations of immunoglobulins correlated with higher percentages of tumor free recipients. Adapted from mAbs. 2014 Jul-Aug;6(4):968-77.
Mice with human immunity and human tumor xenografts
The NSG mouse, NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (005557), is a highly immunodeficient strain proven to support the engraftment of highly enriched human CD34+ hematopoietic stem cells (hHSCs) that develop into functional, terminally differentiated cells capable of innate and adaptive immune responses. These hHSC-engrafted mice are commonly called “humanized” mice, or simply hu-NSG. NSG mice also support the growth of a wide range of human homogenous cancer cell lines and heterologous primary human tumors. This begs the question, “What happens when you engraft a human tumor in a hu-NSG mouse?” Because these mice have functional immunity, you would predict that human leukocyte antigen (HLA) -mismatched tumor cells would simply generate an allogeneic response and would be quickly destroyed. But a number of publications describe ways in which researchers believe tumors evade the host immune system. In this new publication, researchers tested this idea. In the report by Wege et al., irradiated neonatal NSG mice were co-injected with human CD34+ hematopoietic stem cells and HER2+ human breast cancer cells (either SK-BR-3 or BT474) intrahepatically. Multilineage hematopoietic cell engraftment was verified, and HER2+ tumors were found primarily in liver, lung and brain. Tumors were maintained in the majority of these mice for 15 to 36 weeks post-engraftment; only two mice transplanted with the BT474 cell line remained tumor-free. These results demonstrate that hHSCs and HLA-mismatched tumor cell lines can co-engraft.
Immunoglobulins specific for human tumors
One potential limitation of hu-NSG mice is their limited capacity to make human immunoglobulins. Humanized-NSG mice typically can make human IgM antibodies, but in most papers published to date, the hHSC-derived human B cells undergo minimal class switching and produce low quantities of IgG. The recipient mice in the Wege et al. studies were examined for both IgM and IgG, and in agreement with previous publications, most of the co-injected mice produced primarily IgM and very low amounts of IgG. Interestingly, mice in the BT474 tumor-engrafted group that were tumor-free produced more IgM and IgG than their tumor-bearing counterparts. Further, the percentage of mice that survived tumor-free was greatest when IgM was >20ug/ml and when IgG was >10ug/ml. In contrast, none of the SK-BR-3 tumor-engrafted mice were tumor-free, even those mice with total IgG >10ug/ml. Therefore, elevated antibody titer was not necessarily an indication of therapeutic activity, but did indicate the potential for the human B cells to make IgM and IgG when stimulated with engrafted tumors.
When serum from hHSC and tumor cell-engrafted mice was examined for tumor-specific antibodies using ELISA plates coated with either SK-BR-3- or BT474-derived cell lysates, tumor antigen-specific antibodies were detected in 3 of 10 SK-BR-3 transplanted mice and 5 of 13 BT474 transplanted mice. Further probing of the antibodies’ specificities using tumor cell protein lysates and immunoblotting revealed that 8 of 11 SK-BR-3 transplanted mice and 14 of 23 BT474 transplanted mice produced tumor-specific antibodies to multiple antigens. The strongest signals detected were unique and not HER2-specific. Taken together, the human derived B cells in these mice were able to produce tumor-specific antibodies and were capable of Ig class switching.
Antibody-producing plasma cells
Histological examination of spleens from co-engrafted mice identified CD4+ and CD8+ human T cells and CD19+, CD20+ and CD79A+ human B cells. These cells were clustered around CD68+ antigen-presenting macrophages in structures resembling germinal centers found in normal human spleens. The CD79A protein is associated with non-covalent binding to the immunoglobulin heavy chain of the B cell receptor (BCR). A subpopulation of B cells within these cellular clusters also stained with immunoglobulin kappa constant protein (IGKC). The presence of both CD79A+ and IGKC+ cells provides evidence for B cells in the co-engrafted mice that were mature, antibody-producing plasma cells.
The results presented by Wege et al. provide strong evidence that hu-NSG mice co-transplanted with tumor cells can generate human B cells that make tumor cell-specific immunoglobulins. Such mice are a significant advance toward developing a small-animal model that will enable researchers to better understand the complex relationships between human immunity and tumor growth and development. Remaining questions include:
- Can clonal tumor-specific B cells be isolated and used to create hybridomas for monoclonal antibody production?
- Are the antibodies that are capable of tumor clearance truly unique tumor-specific antigens or simply anti-HLA, indicating an allogeneic response?
- If a tumor-specific monoclonal antibody can be produced, will it elicit antibody-dependent cellular cytotoxity and be therapeutic?
The work presented here demonstrates that the hu-NSG platform is well suited to answer these important questions.
