Hu-CD34+ NSG-SGM3 are NSG-SGM3 (NOD.Cg-Prkdcscid Il2rgtm1Wjl Tg(CMV-IL3, CSF2, KITLG)1Eav/MloySzJ, Stock No. 013062) mice engrafted with human cord blood-derived CD34+ hematopoietic stem cells. NSG-SGM3 mice combine the features of the highly immunodeficient NOD scid gamma (NSG) mouse platform with transgenic expression of human IL3, GM-CSF (CSF2) and SCF (KITLG) cytokines for improved development of human myeloid lineages and regulatory T cell populations. hu-CD34+ NSG-SGM3 mice exhibit superior, stable engraftment of diverse hematopoietic lineages for immunology and immuno-oncology research.
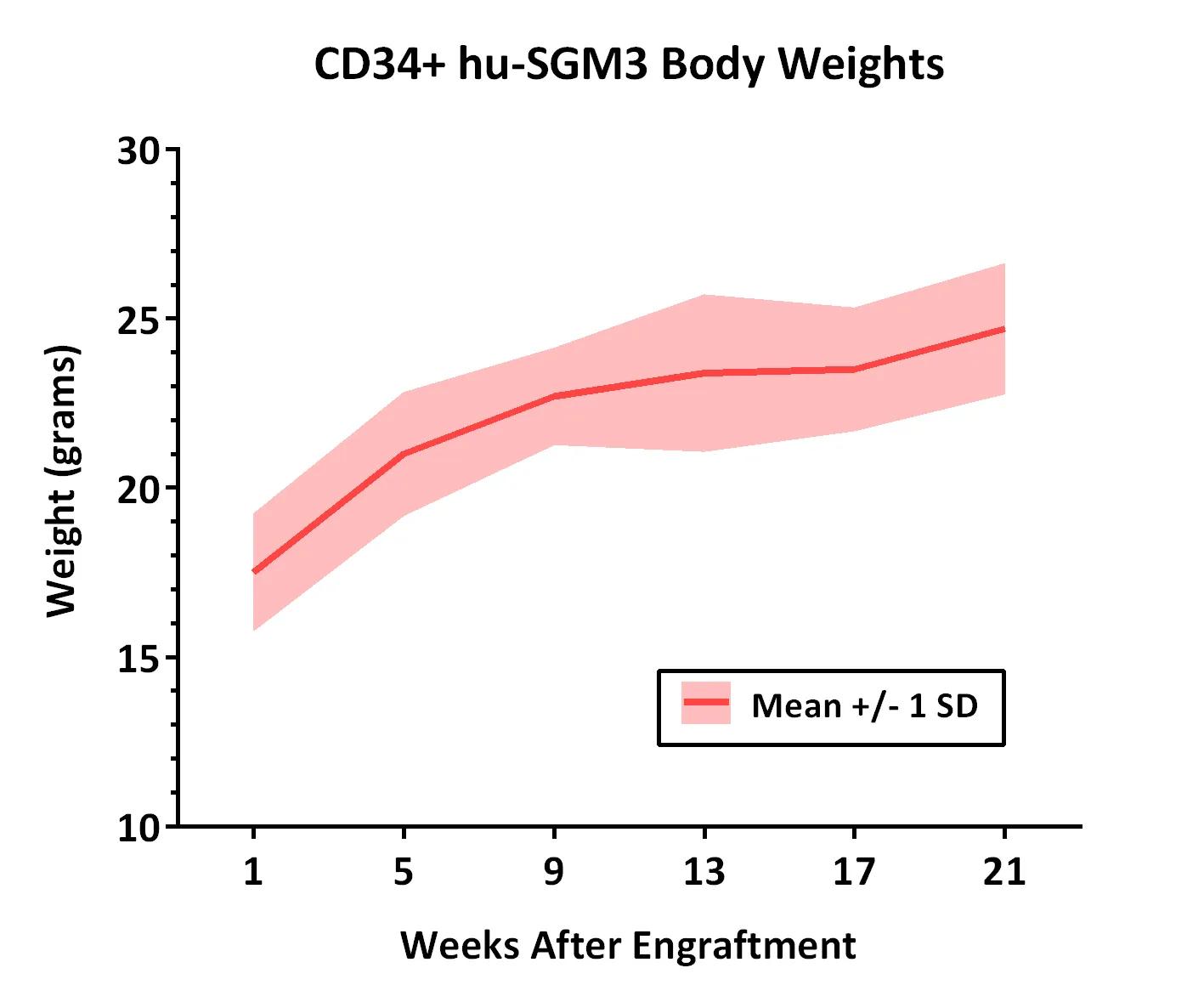 Weights of 10 CD34+ hu-SGM3 mice were measured at different time points after engraftment. All mice weighed were confirmed to be engrafted with >25% hu-CD45+ peripheral blood cells at the standard time point for analysis (16 weeks after engraftment). Weights of CD34+ hu-NSGTM mice are also available.
Weights of 10 CD34+ hu-SGM3 mice were measured at different time points after engraftment. All mice weighed were confirmed to be engrafted with >25% hu-CD45+ peripheral blood cells at the standard time point for analysis (16 weeks after engraftment). Weights of CD34+ hu-NSGTM mice are also available.| Weeks After Engraftment | 1 | 5 | 9 | 13 | 17 | 21 |
|---|---|---|---|---|---|---|
| Mean Weight (grams) | 17.5 | 21.0 | 22.7 | 23.4 | 23.5 | 24.7 |
| Standard Deviation | 1.7 | 1.8 | 1.4 | 2.3 | 1.8 | 1.9 |
Similar to other immunodeficient strains, maintaining NSG-SGM3 mice in high health status (specific pathogen-free) vivaria promotes overall colony health. The fertility of hu-CD34+ NSG-SGM3 mice has not been tested. The engrafted human immune system is not heritable and would not be transmitted by breeding. A small percentage of hu-CD34+ NSG-SGM3 may develop macrophage activation syndrome (MAS). Please see our FAQ below for more details.