Reading a genomic tumor test report
Once the decision to pursue genomic tumor testing has been made, the clinician orders the test and waits for results. Genomic tumor test results can look very different, depending on the laboratory, but most of them contain similar information. This activity uses a mock report formatted to match reports ordered through the MCGI.
Monica is a 53-year-old female with progressive disease after one line of chemotherapy for platinum-resistant ovarian cancer. Her oncologist ordered a large genomic tumor test on the tissue remaining from her initial biopsy to try to identify novel therapeutic targets. Using Monica’s genomic tumor test report, answer the following questions:
Is this patient a candidate for immunotherapy?
No. Monica is not a candidate for immunotherapy. The tumor mutational burden is 5.3 mut/Mb, which is below the laboratory’s cut off of 20 mut/Mb for TMB-high, and MSI status is stable. Tumors that are TMB-high or are microsatellite instable (MSI) have been shown to be more likely to respond to immunotherapy. This information can be found in the table under the Test Results Summary header. PD-L1, another immunotherapy biomarker, was not ordered for this patient and therefore is not included in the report. If it had been, it would have been found in the same place as MSI and TMB results.
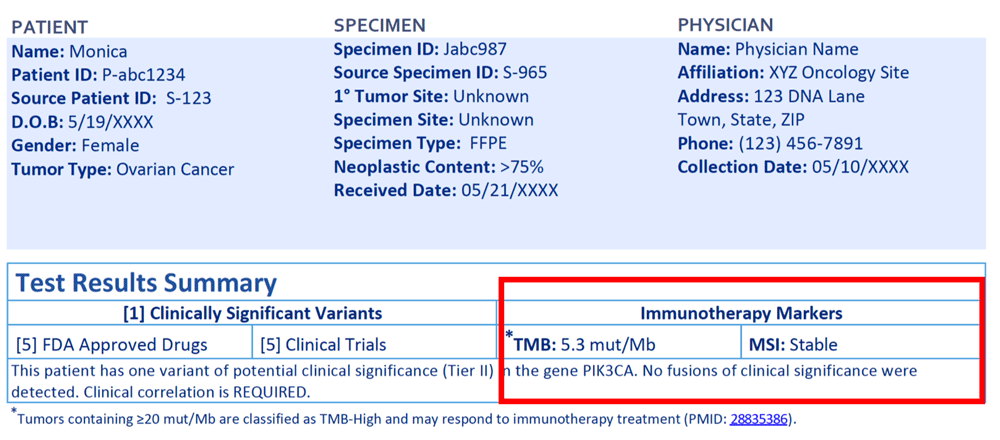
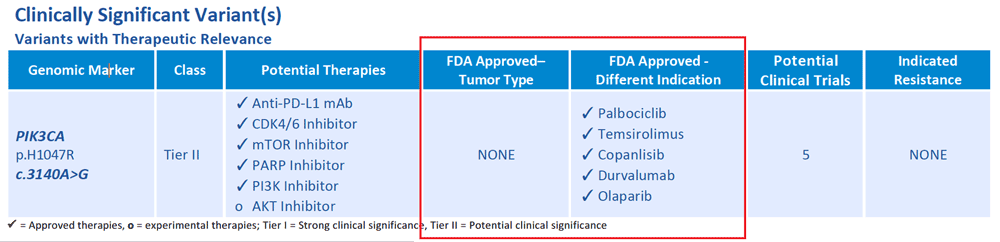
Are there any FDA approved drugs for the patient’s cancer type?
No, there are targeted therapies that have been shown to be effective against PIK3CA variants in tumor types other than ovarian cancer. They are FDA approved in other cancer types, which means they may be accessible to this patient through clinical trials or off-label use.
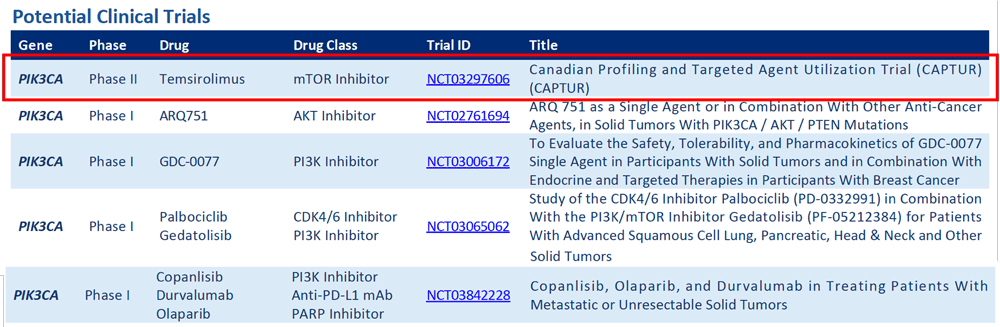
Are there any late stage (phase II or phase III) clinical trials available for this patient?
Yes. There is one phase II trial using an mTOR inhibitor listed, for which this patient may be eligible. Further investigation of the trial’s specific inclusion and exclusion criteria, as well as where the trial is located, is needed to determine if this particular patient would be eligible to enroll and able to access the trial.
Are there any results that are not actionable?
Yes. There are two variants of unknown clinical significance (VUS) listed, one in BRCA1 and one in TP53. VUS should not be used for clinical decision making as there is not enough evidence to determine whether they impact gene function or response to therapy.
