Investigates the molecular mechanisms shaping hair cell architecture in the inner ear
Investigates the molecular mechanisms shaping hair cell architecture in the inner ear
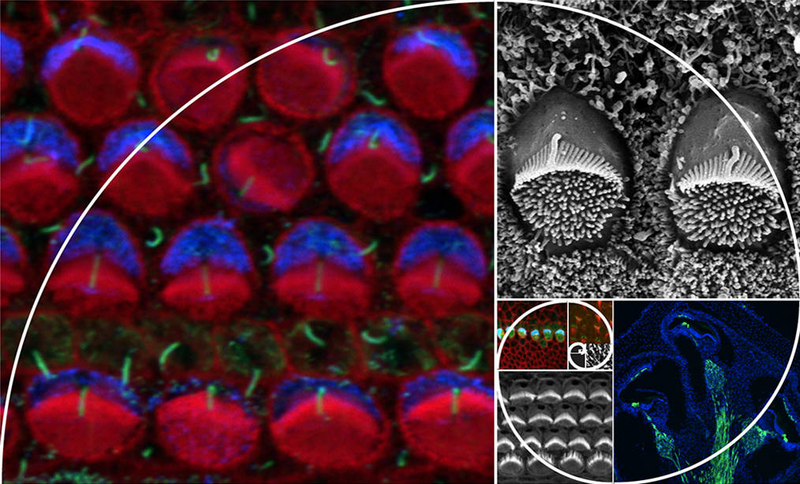
The Tarchini Lab investigates how sensory hair cells in the inner ear detect sound and head movement. As they mature, these cells grow hair-like protrusions that are precisely organized to act together as a motion sensor, converting mechanical input into electrical signals for the brain. Our research identifies molecular programs that govern how this unique cellular architecture emerges and remains stable throughout life. By revealing how sensory function is supported, our findings drive new approaches to prevent or treat hearing loss and balance disorders.
We are hiring postdoctoral associates! Please email a cover letter and CV if interested.
Our long-running interest is to investigate how sensory hair cells in the inner ear develop their stereociliary hair bundle, an apical structure that transduces mechanical stimuli (sound, head movements, gravity) into electrical impulses interpreted by the brain. Our work centers on molecular mechanisms that polarize the apical cytoskeleton, establishing, for example, the graded height organization of stereocilia in the hair bundle. This graded architecture confers directional sensitivity, an essential property for auditory and balance function.
We have identified key factors in this process, such as G protein signaling modulator 2 (GPSM2) and inhibitory guanine nucleotide-binding proteins (GNAI). GPSM2 variants have been implicated in congenital hearing loss in multiple families. GPSM2-GNAI forms an asymmetric complex that acts as an early spatial blueprint to define the edge of the forming hair bundle, and later as a driver of stereocilia elongation. This dual role defines the tallest stereocilia row and helps shape the staircase-like architecture of the hair bundle.
Relevant publications:
Jarysta et al., 2024, Akturk et al., 2022, Jarysta and Tarchini, 2021, Tadenev et al., 2019, Siletti et al., 2017, Tarchini et al., 2016, Tarchini et al., 2013.
Research from our lab and others has clarified that distinct mechanisms polarize and orient the apical cytoskeleton along the epithelial plane in sensory hair cells. Orientation depends on junctional proteins, including core planar cell polarity (PCP) adhesion complexes and the G protein-coupled receptor GPR156. We discovered that GPR156 is critical for establishing proper hair cell orientation in both the cochlea and vestibular organs, providing an etiology for hereditary hearing loss in families carrying GPR156 variants. Notably, GPR156 signals through GNAI independently from GPSM2 to reverse how a subset of hair cells establish their orientation. In the vestibular organs that detect linear acceleration in vertebrates, or in neuromasts that sense fluid flow in fish, this mechanism produces two hair cell populations with opposite orientations. In collaboration with other groups, we have shown how mirror-image hair cell organization and the resulting bidirectional sensitivity contributes to sensory function in mammals and fish.
Relevant publications:
Jarysta et al., 2024, Hughes et al., 2024, Ono et al., 2024, Jia et al., 2023, Kindt et al., 2021, Tarchini 2021.
We have discovered that the GPSM2-GNAI complex sits at stereocilia tips and not only drives elongation during hair cell development, but also plays a critical role in maintaining uniform stereocilia height and the structural integrity of the hair bundle in mature, active hair cells. This line of research addresses how GPSM2-GNAI regulates actin dynamics to preserve lifelong plasticity at stereocilia tips. Ongoing work explores whether tip proteins exert a protective role in response to aging and environmental stressors such as noise exposure.
Relevant publication:
Hartig et al., 2024.
In emerging projects, we are exploring how polarity cues shape the differentiation of glial-like supporting cells in the auditory epithelium. These cells share common progenitors with hair cells and perform multiple non-sensory roles essential for hearing. Intriguingly, supporting cells can de-differentiate and act as progenitors to replenish injured hair cells, although this ability is rapidly lost in mammals. Supporting cells are best known for providing rigid structural support for cochlear mechanics, a role that requires dramatic morphometric changes during cell differentiation. This remodeling remains largely understudied and interestingly appears to rely heavily on cytoskeleton polarization. A better understanding of supporting cell biology is essential to unlock their promising potential as therapeutic targets for hearing restoration.