Anti-aging interventions
III.A. Diet restriction
Diet restriction: retards aging in fat hyperinsulinemic mice
Using the C57BL/6J Lepob/Lepob (ob/ob) mouse, which produces no leptin, we investigated the relationship between body fat and diet restriction (DR). Under DR, we reduced the fat of ob/ob mice to 48% of their total body weight, which is still about 4 times the fat composition of ad lib-fed normal controls. Nonetheless, these ob/ob mice lived as long as diet restricted genetic controls (Harrison et al., 1984, 1991). These results demonstrate that the extremely large stores of fat in diet restricted ob/ob mice — and their high levels of circulating insulin — do not prevent them from gaining the full benefit of DR.
Diet restriction: retards stem cell aging
Age-related loss of hematopoietic stem cell (HSC) function may be a major cause of anemias and can also lead to other defects such as those related to immune function. Treatments to improve loss of HSC function exist, but unfortunately, such treatments may increase the incidence of cancer. Because DR delays the expression of cancer, we investigated whether it could provide a new treatment model that successfully delays both the age-related loss of HSC function and the increase of cancer incidence. We discovered that DR does mitigate both age-related phenotypes, and that this effect is strain dependent (Ertl et al., 2008; Ertl and Harrison, 2008).
Diet restriction: retards aging in a 4-strain cross of mice
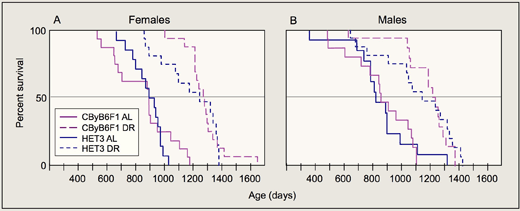
We studied DR in UM-HET3 (HET3) mice. These mice are the first generation offspring of CByB6F1/J and C3D2F1/J parents and are used in the Interventions Testing Program (ITP; see below). In the first report of DR in a structured, segregating heterogeneous population, we observed essentially the same increases in mean and maximum lifespan as found in CByB6F1/J hybrid positive controls (Figure 1; Flurkey et al., 2010). All of the diet restricted HET3 mice in our study survived past the age when the ad lib-fed control HET3 mice began to die; in fact, the first age-related death among diet-restricted HET3 males did not occur until half the ad lib-fed HET3 males had already died. These results are important for 2 specific reasons: First, we showed that DR increases lifespan in mice across the broadest range of genotypes tested to date. Second, we did not see the early deaths sometimes observed for individual diet restricted mice within an inbred strain.
III.B. Intervention Testing Program (ITP)
As part of a 3-institution project funded by the National Institute of Aging, we are testing simple chemical interventions administered through food to determine if they retard aging and increase life span in mice. We consider an intervention successful if it increases insulin sensitivity and reduces chronic inflammation and susceptibility to oxidative damage, all of which are well-known indicators of retarded aging. We will test the most successful interventions in combinations at all centers.
Mice in the study are the 4-strain cross UM-HET3 (HET3) mice, the first generation offspring of CByB6F1/J and C3D2F1/J parents, first used by Miller et al. (1999) to study life spans. Thus, the tested populations are reproducible and represent great genetic diversity, helping to assure that results are not due to the responsiveness of a single genotype but more broadly represent laboratory mice in general. We are including a group of diet restricted HET-3 mice as positive controls because diet restriction is the "gold standard" for treatments that retard aging and increase life span.
Although one of the most important criteria of retarded aging is increased maximum life span, the ITP also includes longitudinal monitoring of individual biological systems that can be conducted without harm to the mice: proportions of blood T cell subsets, voluntary activity, plasma IGF, glucose, insulin, and thyroid hormone. We also test pathologies at death.
Rapamycin treatment that extended life span (Harrison et al., 2009) is especially promising for 2 reasons: First, successful treatment started when mice were 600 days old — late middle-age. Results are equivalent to increasing the life expectancy of a 60-year-old human being by 10 years. Second, the size of the life span increase suggests that rapamycin treatment delayed an aging mechanism in the HET3 mice. If rapamycin had merely delayed the onset of several aging-related diseases, the life span increase would have been smaller. Our rapamycin results are especially interesting from a clinical perspective, because we expect that humans are most likely to consider anti-aging treatment when they reach middle age, and our study shows that such treatment could be successful.
Read more about the ITP rapamycin study…
In the Harrison Lab only, we also conducted several studies that extended, but were not specifically part of, the 3-site ITP (Flurkey et al., 2010). Using HET3 mice and the same laboratory environment as in the ITP, we tested 4 treatments: N-acetyl cysteine (NAC) started at seven months, and aspirin, nitroflurbiprofen (NFP), 4-hydroxy phenyl N-tert-butyl nitrone (4-OH-PBN), and nordihydroguaiaretic acid (NDGA), all started at 16–18 months. Only male HET3 mice receiving NAC had significantly increased life span, and this may have been due to treatment-related, inadvertent DR. The other agents had no significant effects on lifespan.
III.C. Corticosterone
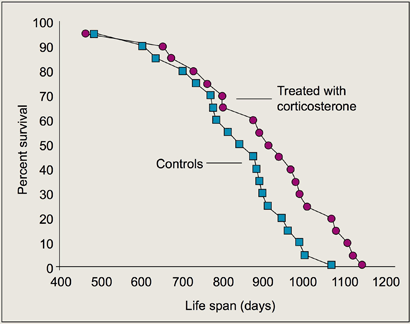
Two conditions associated with corticosterone elevation — DR and hypophosectomy — are known to increase life span. DR elevates circulating corticosterone, and lifelong corticosterone supplementation is a critical component of rodent hypophosectomy studies in which life span was extended. Thus, we were interested in determining the effects of chronic corticosterone treatment in normal mice. Results show that, in B6CBAF1 males, chronic physiological elevation of corticosterone increases maximum life span by about 10% (Figure 2). We conclude that the effects of DR on life span could be partly, but not entirely, mediated by chronic evaluation of circulating corticosterone.
III.D. Environmental enrichment and husbandry
Igloos and tunnel cages: Using our 4-strain cross mouse model (CZECHxMOLF F1 females x WSBxSKIVE F1 males), we are studying the effects of social dynamics by monitoring the effects of igloo-like enclosures and tunnel cages (Figure 3). Igloos, which have room for up to 2 mice, provide shelter and isolation. The tunneled cage structure comprises 2 shoebox cages connected by a tunnel. Although the housing density (number of mice per unit of cage space) remains the same as with standard housing, mice have extra space in which to move around. This housing system has 2 distinct advantages: First, cleaning cages is greatly simplified. Caretakers simply guide all the mice through the tunnel into one cage; disconnect the empty, dirty cage; and attach a clean cage. They never have to physically pick up mice and move them to a new cage, thus alleviating stress on both the mice and the caretakers. The second advantage relates specifically to life span studies. Mice are social animals and thrive best when housed with other mice. And as mice in a study die, single survivors should be rehoused. Rehousing with new mice, however, is disruptive and stressful, especially for most males. With larger groups in tunnel cages, less rehousing is required. When the number of mice in the tunnel cages is low enough for one cage, all mice are simply housed together in a single cage. Experiments are ongoing.

