For the last decade, Robert Burgess, Ph.D., a professor at The Jackson Laboratory (JAX), has studied Charcot-Marie-Tooth disease, a degenerative nerve disorder. He experiments with mice that model the disease’s genetic origins.
Occasionally he will get an email or a call from someone who’s found his work on the Internet: Can he help a loved one who’s been diagnosed with the disease?
“No,” Burgess typically replies with regret. “I can’t even help most of my mice,” he says.
Once Scott called me, it's like, ‘This makes total sense. We should do this project.’
Such was the case when Dr. Stephen Fletcher, a pediatric neurosurgeon from Houston, emailed him in the summer of 2013, asking if Burgess could help with his granddaughter, Caroline. As a toddler Caroline had been diagnosed with a form of Charcot-Marie-Tooth known as type 2D (CMT-2D), and her case was unusually severe.
Charcot-Marie-Tooth is a group of peripheral nerve disorders that cause muscle weakness and wasting in the feet, legs, hands and arms, and reduced sensation in the limbs.
The disease is robbing Caroline of muscle strength, preventing her from standing or walking, restricting the use of her hands, and even making it difficult to breathe.
“It was a compelling story,” Burgess recalls, “(I said) I'm really sorry to hear about this, but my lab works exclusively on mice, and I don't have any clinical practice or facilities. We have the long-term goal of understanding this disease and developing a treatment, but that's a long-term goal.’"
Burgess suggested that Fletcher contact scientists at medical centers who might be involved in clinical trials of potential Charcot-Marie-Tooth therapies. He thought that would be the end of his involvement.
A few months later, one of those clinical scientists, Scott Harper, Ph.D., a principal investigator in the Center for Gene Therapy at the Research Institute at Nationwide Children’s Hospital in Columbus, Ohio, received an email from Fletcher. As Burgess had done, he was ready to refer Fletcher to someone else.
"My initial impression was to simply inform him that I wasn't working on CMT, was busy with several other projects, and didn't think I would have time to start a new one from scratch,” says Harper, who researches muscular dystrophy. “However, I wasn't familiar with the form of the disease his granddaughter had, CMT-2D, and I figured that before I responded to him, I should at least understand exactly what it was I was refusing to consider.”
Harper researched the disease online and came upon some intriguing scientific papers by Burgess describing CMT mouse models he had identified and characterized.
“After reading Rob's work, I came to realize that many of the strategies we had been developing to treat dominant muscular dystrophy could be applied to CMT-2D,” Harper recalls. “He had the tools and expertise needed to make this project happen.”
Harper called Burgess, and the two quickly bonded. They discovered they were both from the Greater Tri-Cities region of Central Michigan and had studied at rival schools in the state, Harper at the University of Michigan as a graduate student and Burgess at Michigan State University as an undergraduate.
More importantly, their conversation left both men thinking they might be able to help Caroline after all. The pair realized that each scientist held half of a potential solution for her particular form of Charcot-Marie-Tooth.
Harper had developed viral vectors as a tool for gene therapy while Burgess had developed mice that modeled CMT. The vectors are viruses like influenza and many other familiar — and unwelcome — causes of disease. But instead of being disease agents, these viruses are custom-made to combat disease and work like UPS trucks to deliver treatments.
“I could have made the mouse, but I couldn't have done anything with it,” Burgess says. “Scott could have developed a gene therapy vector, but would have never known if it workedin vivo (in a living organism). Once Scott called me, it's like, ‘This makes total sense. We should do this project.’"
And so they are. They have been working together for the last two years.
A third scientist Fletcher contacted, Anthony Antonellis, Ph.D., associate professor of Human Genetics and Neurology at the University of Michigan Medical School, is also collaborating with them. Antonellis has performed important cell-culture studies of Caroline’s disease and has worked with Burgess previously on mouse models.
Just as Fletcher’s determination to find hope for Caroline sparked this collaboration, his philanthropic support has fueled its growth. Fletcher’s gifts to support Burgess’ research (as well as the work of other scientists studying CMT) illustrate the power of philanthropy to accelerate scientific research and, in particular, to serve as a catalyst for innovative research projects that might otherwise not get off the ground.
Charcot-Marie-Tooth is named for the three physicians who first described it in 1886. There are no treatments to stop or slow its progression in the 2.8 million people who have the disease.
Even for the type 2D form of the disease, Caroline’s symptoms presented earlier and more severely than is typical.
“By the time she was 11 months I knew something was up,” says Fletcher. “I started noticing some weakness in muscle groups. We thought she was getting worse. As time went on we noticed her hands weren’t working very well. I think she’s got progressive problems because she can’t gain weight due to atrophy of her muscles.”
More recently Caroline has had trouble breathing, requiring multiple stays in the hospital. “She lost a nerve that makes her diaphragm work,” Fletcher says. “That’s why she’s had so many pulmonary problems.”
Analysis of her family’s DNA revealed that Caroline alone carries a unique genetic mutation responsible for the disease. The mutation prevents her peripheral neurons – nerve cells connecting the brain and spinal cord to the rest of the body – from sending vital electrochemical signals to her muscles.
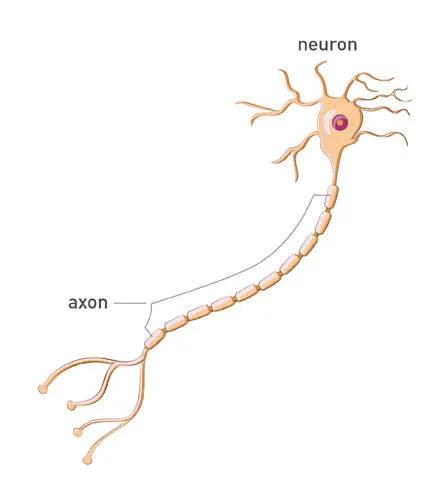
Caroline lacks part of a gene that instructs cells to make glycyl-tRNA synthetase, or GARS, an enzyme essential for protein production. A malformed GARS enzyme is believed to be toxic to axons, the long nerve cell fibers that transmit signals to motor and sensory cells in the limbs and other parts of the body.
“You can literally think of this as electricity,” Burgess says. “If you degrade the axon, your connection is gone. This is just like cutting the wire between the fuse box and the lamp.”
Caroline has one good copy of the GARS gene and one mutated copy.
“Unfortunately the mutation in the bad copy overrides the good gene,” Harper explains. “We think that reducing or eliminating the bad GARS gene will allow her good copy to function normally, and could offer a treatment for her disease.”
In the coming months Burgess and Harper will attempt a gene-silencing strategy in “humanized” mice that are being engineered to contain the exact genetic mutation underlying Caroline’s disease.
“This is the height of personalized medicine,” Harper says.
Burgess will inject the mice with Harper’s viral vectors, altered to deliver small, specific pieces of RNA, the molecule that translates DNA into instructions for making proteins. The vectors insert the micro-RNA into peripheral neurons and other cells, where it will match up with, and disable, faulty RNA.
The challenge with this “RNA interference” technique will be to silence the genetic instructions for making the faulty GARS enzyme without silencing the code for making normal GARS, because survival without GARS is unlikely, Burgess says
If the mouse studies establish proof of principle that the technique works, the next step will be to determine safety through toxicology studies in mice and possibly larger animals, Harper says. Then investigators can apply to the Food and Drug Administration for a clinical treatment plan for Caroline, most likely to be carried out at Harper’s institution.
This is the first time that people have tried this.
“The Center for Gene Therapy at Nationwide Children’s Hospital, led by Dr. Jerry Mendell, is arguably the world’s leader in translating gene therapy for neuromuscular disease,” Harper says.
Meanwhile, he and Burgess are proceeding cautiously.
“This is the first time that people have tried this,” Burgess says. “We're doing our absolute best to do this right and get all the ducks in a row first.”
Harper says his own standard for proceeding to the clinic is, “Would I be comfortable delivering this therapy to my own children?”
Similar gene therapy approaches for other diseases are in clinical trials in the United States and Europe, and they should “help pave the way for translating the work Rob and I are doing,” Harper says.
“After decades of development, and after clearing some roadblocks, we are now finally starting to realize the great promise of gene therapy,” he says. “Importantly, we’ve learned a number of lessons from these early studies that are now finally reaching the clinic, and this gives me great confidence that we will be able to get there with our strategy as well, although I expect it will take time.”
Caroline, now 4 years old, is undefeated by her illness and its daily limitations.
Though she can’t walk, she zips around in a motorized wheelchair (“She calls it her magic chair,” Fletcher says.) She enjoys playing with her fraternal twin, Henry, who is unaffected by CMT, and other relatives and neighborhood friends.
“She doesn’t think she has a problem because she gets out and does stuff with them,” Fletcher says. “She can play. She’s very good and able to adapt to things. Nothing seems to impede her.”
As a grandfather, Fletcher is hopeful for a treatment, but as a physician, he knows how much time, money, work and technology are required to translate a theory into a therapy.
That mouse model they’ve created... I think they’re the go-to guys.
“Bench to bedside, that’s a difficult thing in medicine,” he says. “The clinical application of research is a totally different ballgame.”
He believes it may be too late to reverse the muscle loss Caroline has suffered but thinks gene therapy may be able to stop her disease from progressing.
“I’d like to see her live a long time,” he says. “She’s smart. She’ll have something to contribute.”
If relief for Caroline is possible, Fletcher believes the trio of scientists he has catalyzed on her behalf – Burgess, Harper and Antonellis – will be the ones to deliver it.
“I haven’t met them personally, but I love the hell out of them,” he says. “They are three researchers who work well together, but don’t have egos. That mouse model they’ve created, and what they’ve published in the last few years, I think they’re the go-to guys.”