Studying Complex, Systemic Immune Responses in Humanized Mice
Blog Post | February 14, 2022
Although many mammals have immune systems that share important characteristics with humans, certain immune cell types and phenomena are unique to us. As a result, it has been difficult to design and perform empirical studies that will help researchers accurately predict clinical responses to therapeutic drugs, infectious diseases, cancers, and other stimuli that can trigger an immune response. In particular, system-wide immune disorders such as cytokine release syndrome (CRS) and downstream graft vs. host disease (GvHD) have remained incredibly challenging to study, prevent, and treat. In vitro studies don’t capture the cascading effects that the immune system can have on a wide range of tissues, while in vivo studies in animals such as rodents and non-human primates have historically failed to predict toxicities linked to unique human immune cell types (Eastwood et al. 2010).
To solve these issues The Jackson Laboratory (JAX) has developed a versatile toolset of mouse models capable of supporting extensive functional components of the human immune system. These mice provide a preclinical research platform that yields sensitive, reproducible results to screen drug candidates for inflammatory responses. The platform also offers insight into the progression of immune disorders and the potential efficacy of drugs meant to treat them.
Humanizing Mice for Immunology Studies
To create our humanized mouse platform for studying T cell based immune responses, we engrafted immunodeficient mice (NOD-scid IL2rgnull, or NSG) with peripheral blood mononuclear cells (PBMCs) from human donors. PBMCs include a variety of immune cells, but this engraftment model is dominated by T cells allowing us to recreate a human T cell mediated immune response in the mice.
In validation studies, we found that we could reliably induce a dose-dependent CRS response in these humanized mouse models using compounds known to cause CRS clinically, and that there was no CRS response to immunotherapies known to be clinically safe. Our results also captured the variation in cytokine release observed in the individual human PBMC donors, with greater sensitivity and more reproducible results than in vitro assays performed with cells from the same donors (Ye et al. 2020).
Examining How CRS Affects Organs
CRS occurs when the immune system overreacts to a stimulus such as a drug or disease. Activated white blood cells flood the body with an excess of cytokine signaling proteins, which activate more white blood cells and create a dangerous snowballing effect that can result in fever, organ failure, and even death.
With our humanized mouse model, we have been able to measure not only the levels of various human cytokines released into the bloodstream following drug administration, but also observe the downstream effects these cytokines have on the body over time. We observed changes in body weight, and we collected serum to analyze kidney and liver enzyme levels. We also collected tissue samples from the liver and lungs to perform histopathology studies using hematoxylin and eosin (H&E) staining, Caspase 3 staining, and single cell necrosis detection in hepatocytes.

Figure 1. Histopathology results of H&E staining of lung tissue. Human PBMC engrafted NSG mice were treated with anti-CD28 mAb and TGN1412 analogue drugs, both of which are known to induce cytokine release. Purple staining in the experimental tissue samples indicates high rates of cell mortality.
As expected, based on cytokine release levels, humanized mice treated with compounds known to cause CRS showed lowered body mass and experienced severe organ damage that reduced functionality. We can continue refining these studies to offer a better understanding of how factors such as dosage variation can alter downstream organ effects of CRS.
Modeling GvHD Progression
We also created humanized mice to model GvHD, which affects entire organ systems and can develop from CRS that goes untreated for too long. We generated these models by treating NSG mice with irradiation and then engrafting them with human PBMCs—a process similar to our other studies. However, it was important that the PBMC donors for this particular study had already been pre-characterized specifically for their GvHD response.
We observed that the progression and severity of GvHD in humanized mouse models is specific to individual PBMC donors and closely mirrored the diversity and range of severity of GvHD responses observed in human transplant recipients. This indicates that, as with CRS, this platform could someday be used to help make individualized predictions about how patients’ immune systems will respond to treatment.
We performed an initial study to demonstrate the utility of this platform for preclinical testing of new drugs meant to treat immune transplant complications like GvHD. We used the model to compare Abatacept, an immunomodulator known to slow the progression of GvHD, to high and low doses of a bispecific antibody designed to accomplish the same thing.

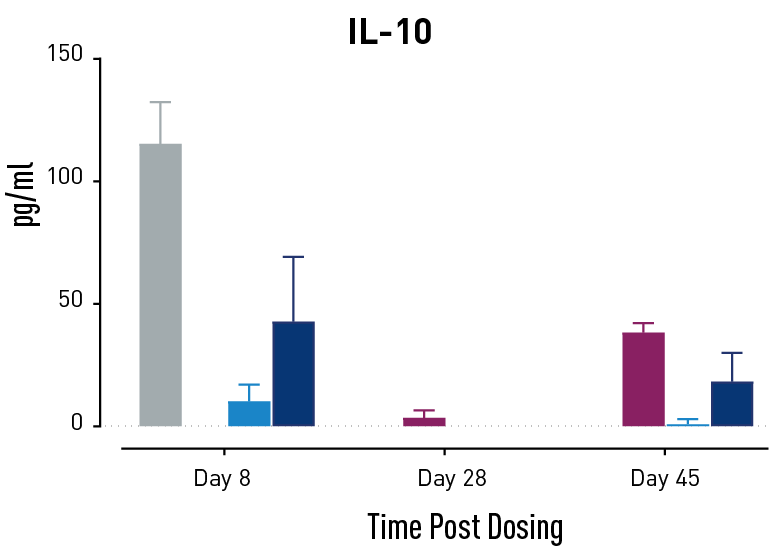

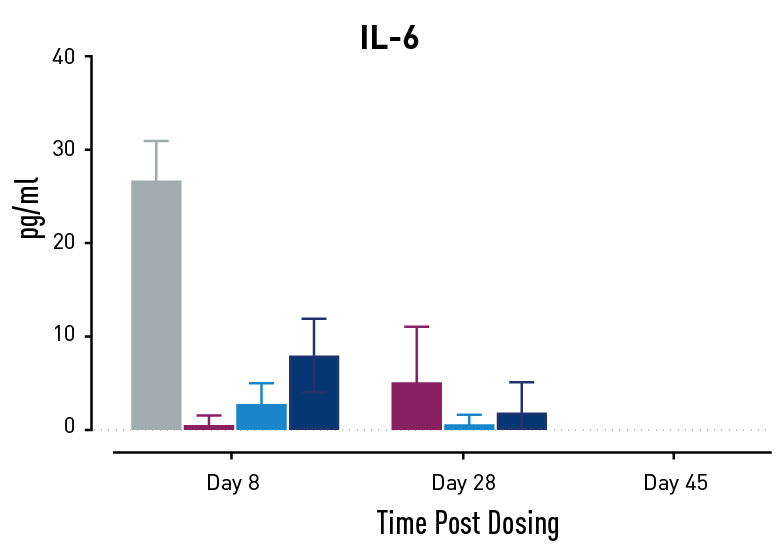

Figure 2. Measurements of cytokines during GvHD progression. Human PBMC engrafted NSG mice modeling GvHD were treated with Abatacept or with a low or high dose of a bispecific antibody. Mice treated with high concentrations of the bispecific antibody showed less cytokine release than those treated with Abatacept on study days 28 and 45.
In humans with GvHD, Abatacept decreases T cell expansion and cytokine release and improves survival. Compared to controls, both Abatacept and the bispecific antibody doses demonstrated all three of these effects. The high dose of the bispecific antibody performed better than Abatacept in the latter weeks of the study; this result demonstrates that this humanized mouse platform can provide nuanced information about the efficacy and mode of action of any new drug intended to treat GvHD.
Visit our CRS Evaluation Study page to learn more about JAX’s cutting-edge humanized mouse platform for studying immune responses, from bench research to drug pipelines.
References
Eastwood D, Findlay L, Poole S, Bird C, Wadhwa M, Moore M, Burns C, Thorpe R, Stebbings R. Monoclonal antibody TGN1412 trial failure explained by species differences in CD28 expression on CD4+ effector memory T-cells. Br J Pharmacol. 2010 Oct;161(3):512-26. doi: 10.1111/j.1476-5381.2010.00922.x. PMID: 20880392
Ye C, Yang H, Cheng M, Shultz L, Greiner D, Brehm M, Keck J. A rapid, sensitive, and reproducible in vivo PBMC humanized murine model for determining therapeutic-related cytokine release syndrome. FASEB J. 2020 August 09;34:12963-12975. doi: 10.1096/fj.202001203R
