That Feeling When You Can’t Reject the Null Hypothesis
My first foray into research was as an undergraduate tech studying how environmental pollutants like dust, ozone, and smoke affected lung function and development (see for example, Kott et al, 2002, J Appl Physiol). My project was to measure changes in airway structure from stained tissue sections. The majority of those minimum wage hours (starting well after tissue collection, tissue processing, and paraffin embedding) went a little like this:
Protocol
- Cut and mount sections on slides; stain with H&E, coverslip.
- Take digital images of micrometer scale and 2-3 sections per airway per lung lobe per animal at 10x magnification. Save images to Zip disk.
- On a computer, open micrometer scale image in NIH Image, and convert pixels drawn to uM length. With a computer mouse, trace smooth muscle surrounding airway.
- Repeat steps 1-3 one thousand times, or until eyes fall out/carpal tunnel sets in.
Never mind that my data did not make it into the previously mentioned paper. Despite my efforts, there was no difference in smooth muscle volume between the two groups I was testing.
Occasionally when a researcher contacts me in JAX Technical Information Services (TIS) for breeding advice, I recall those days on the bench where I worked my tail off trying to find a difference that didn’t exist; I had nothing to show for all my hard work except a sore wrist and a very sore attitude. In TIS, we answer questions from mouse researchers like “should I backcross my cre and lox mouse strains to a pure genetic background first and then make the knockout? Or should I create the knockout on the mixed genetic background first, gather some preliminary data, and then backcross?” I usually argue for the latter: do they want to spend years backcrossing before finding out that their hypothesis is wrong or their mutation is lethal?
Enter IMPC, to the Rescue
Now imagine if a knockout mouse model for your favorite gene just magically landed in your lab. Imagine that it’s already on a pure, commonly used genetic background (making control selection a breeze). Imagine knowing ahead of time whether it is embryonic lethal, infertile, or has defects in major organ systems. Think of all the time and resources you would save in this scenario!
As it happens, an international consortium of geneticists is making this dream a reality. Members of the International Knockout Mouse Consortium (IKMC) and the International Mouse Phenotyping Consortium (IMPC) have embarked on a massive endeavor to systematically knock out every gene in the mouse genome, to characterize the mutants, and to make all of the materials and data publicly available to researchers worldwide. A collection of their initial findings looking at embryonic lethal genes from the first ~1800 genes completed thus far was published in the September 22, 2016 issue of Nature.
A reverse genetics approach of this scale could generate upwards of tens of thousands of new hypotheses for researchers worldwide. Given the 20,000+ genes in the mouse genome, this task would be impossible to achieve without collaboration between nearly 20 institutes around the world, including The Jackson Laboratory, and implementation of precise, high-throughput technologies, such as high-resolution 3D imaging of embryos and CRISPR/Cas9 mutagenesis to generate new knockout models. Considering the amount of time it would take to analyze a handful of histology sections or to generate knockout mice using conventional methods, the implementation of newer, high-throughput technologies will certainly expedite data collection and improve data accuracy.
Phenotypic characterization of the mice produced by this project started just a few years ago, with many more live, mutant mice and data coming through the pipeline. The wealth of information that has already been generated has the potential to impact your research directly and immediately. Below are some ways you can use this resource now, even if mice that carry mutations in your favorite gene are not yet available, or are only partially characterized.
Highlights from the knockout mouse phenotyping resource:
- Standardized methods, including exon deletions for alleles generated by CRISPR/Cas9 mutagenesis, are used to target genes on well-characterized C57BL/6N mouse genetic backgrounds (such as JAX’s C57BL/6NJ, 005304). Because this genetic background is so commonly used, you may already have a disease model that you can cross to these mutants or to use as experimental controls.
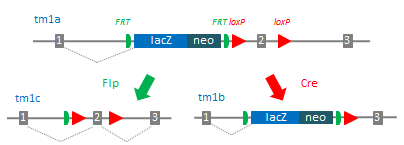
Examples of targeting vectors generated by IKMC. When a mouse mutant for your favorite gene (tm1a) is bred to a Flp mouse strain (green), the FRT sites recombine to generate a conditional allele (tm1c). Alternatively when tm1a is bred to a Cre strain (red), the loxP sites recombine to generate a combination knockout/lacZ reporter allele (tm1b).
- Sequences of independent clones available at GenBank to aid in generating genotyping assays and to allow full transparency for the mutation being analyzed.
- Standardized phenotyping pipeline with linked protocols. Although the mutants within the database are being made and characterized by different IKMC organizations, they are all using mutually agreed upon protocols to characterize their mice. Because the phenotyping protocols are standardized, phenotypes between different mutants can be compared with greater confidence that any differences observed are real, rather than protocol-dependent artifacts.
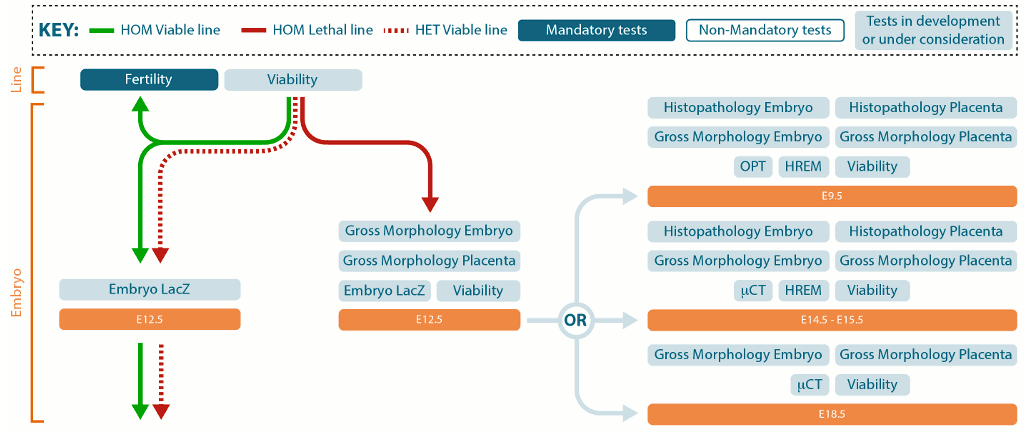
The phenotyping pipeline shows the data you may expect for each time point, and can serve as a resource if you are looking for a protocol to characterize your own mutants.
- Rigorous QC and statistical data analysis compiled into one web-accessible database. The ability to manipulate and export available data allows you to generate new hypotheses.
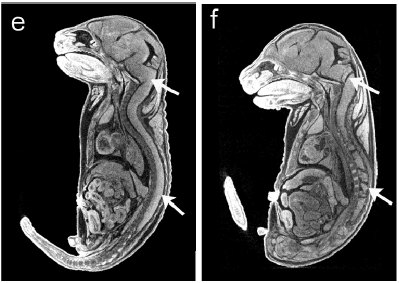
Access data, such as 3-D images of embryonic lethal mutants in the IMPC database. This particular online tool allows you to simultaneously dive through WT (left) and homozygous mutant (right) embryos in 3 anatomic orientations. Exporting data from several embryos into other digital analysis programs can allow you to accurately measure morphometric differences across multiple organs in an entire embryo. Carpal tunnel, be gone!
- Search by gene to determine potential biological function. A major goal of the consortia is to target genes that have gone largely unstudied. If your favorite gene isn’t already in the pipeline, you can:
- Nominate the gene at JAX KOMP.
Acknowledgements
Thank you to our JAX researchers, particularly Steve Murray, Ph.D., Jim Denegre, Ph.D. and Candice Baker, Ph.D., for their contributions to the IKMC, IMPC, and to this blog article.
Resources
Dickinson ME, et. al. High-throughput discovery of novel developmental phenotypes.
Nature. 2016 Sep 14;537(7621):508-514.
IMPC website to access the standardized data: