New findings may usher in a new era of treatments preserving long-term ovarian function and fertility in women undergoing cancer treatment.
Researchers at The Jackson Laboratory (JAX) have identified a master regulator of cancer treatment– induced ovarian failure and infertility, opening up potential therapeutic avenues for much-needed treatments to preserve a woman’s long-term ovarian function and ability to have children despite toxic cancer treatment.
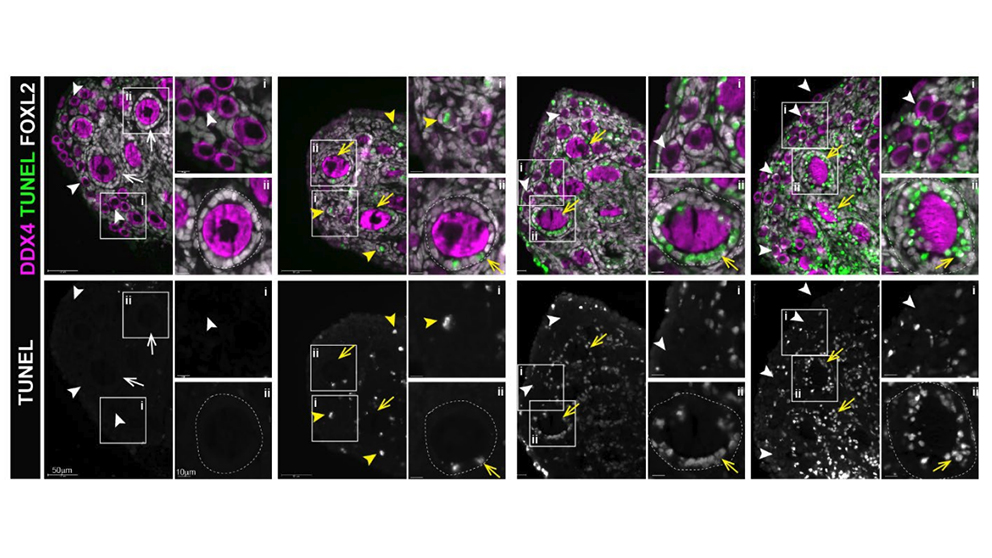
Using mouse models, Ewelina Bolcun-Filas, Ph.D., a JAX associate professor whose lab studies fertility, and her colleagues found that the CHEK2 protein is responsible for coordinating the elimination of primordial follicles containing immature oocytes, which are the cells in the ovaries that go on to become eggs, after they are damaged by chemotherapy drugs and radiation therapy. Further, they discovered that eliminating CHEK2 with an inhibitor that blocks its activity preserves ovarian follicle reserve—the finite capacity of the ovaries to provide eggs capable of fertilization—following these treatments. The findings were published in Science Advances.
New findings about CHEK2
“We’ve known for years the harmful effects that chemo and radiation can have on women’s reproductive health, but treatment options are still limited,” says Bolcun-Filas. “Our new findings about CHEK2 suggest that the protein is an attractive target for the development of treatments that protect the ovaries and primordial follicles, and ensure reproductive health and the likelihood of a successful pregnancy for women cancer survivors.”
Primordial follicles constitute the long-term reserve of immature eggs and thus are a major source of the female hormones that are responsible for development and regulation of the female reproductive system and women’s overall health. Primordial follicles decrease naturally until menopause, but environmental factors such as diet, smoking, pollution and certain medical treatments can also harm them and accelerate menopause.
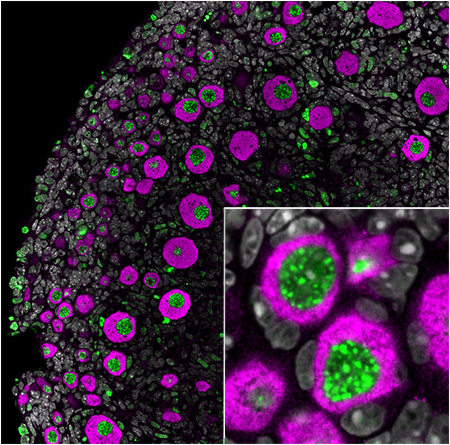
When ovarian follicles grow to produce an egg, it triggers the release of hormones from the ovaries. If a young woman undergoing cancer treatment loses all her follicles, the ovaries will stop producing female hormones and the patient will enter premature menopause. Women who experience ovarian failure at a younger age may face an increased risk of health problems, such as heart disease, stroke and cognitive decline, typically associated with menopause occurring later in life.
The chemotherapy connection
Chemotherapy and radiation therapy kill cancer cells by inducing DNA damage, which is detrimental to fast-dividing cells such as tumor cells. These treatments, however, can also damage healthy cells, including oocytes. The most lethal type of DNA damage—double-strand breaks, which occur when both strands of DNA in a cell are severed—leads to the activation of CHEK2. This type of DNA damage is the major trigger of radiation- and chemotherapy-induced elimination of primordial oocytes. Due to the DNA damage response coordinated by CHEK2, primordial oocytes are eliminated before they can repair these breaks.
Through genetic studies, Bolcun-Filas and her colleagues revealed that two targets of CHEK2—p53 and TAp63—are activated by a class of chemotherapy drugs known as alkylating agents that have been shown to contribute to oocyte elimination. Alkylating agents interfere with a cell’s DNA and inhibit cancer cell growth.
Because healthy primordial oocytes are sensitive to DNA damage-induced apoptosis (a form of programmed cell death), blocking the DNA damage response should allow more time for DNA repair and could successfully prevent follicle loss. Bolcun-Filas and her team found that shutting down CHEK2 protected greater than 90% of follicle reserve in female mice treated with cyclophosphamide and cisplatin, two common chemotherapy drugs that are toxic to the ovaries, and protected primordial oocytes from destruction by other frequently used chemotherapies, including doxorubicin, etoposide and mafosfamide.
Insights into CHEK2 inhibition
The researchers also discovered that blocking CHEK2 activity in the ovaries with the investigational drug AZD7762, a dual CHEK1/2 inhibitor that sensitizes cancer cells to chemotherapy and radiation, preserved ovarian reserve after these treatments. Bolcun-Filas tested three other CHEK2 inhibitors that showed no protective effect.
“We were able to show that AZD7762 can prevent radiation and chemotherapy induced primordial oocyte elimination,” says Bolcun-Filas, “which suggests the importance of developing selective inhibitors specific to CHEK2 as potential treatments in preventing eradication of ovarian follicle reserve.”
Bolcun-Filas says that CHEK2 inhibition may be more suitable for women who prefer to forgo ovary removal and freezing of their eggs for reproductive purposes or for women who have already gone down that route but want to reduce their risk of premature menopause and other reproductive aging complications.