Constructing a map of the human body at the highest resolution
Research Highlight | July 18, 2023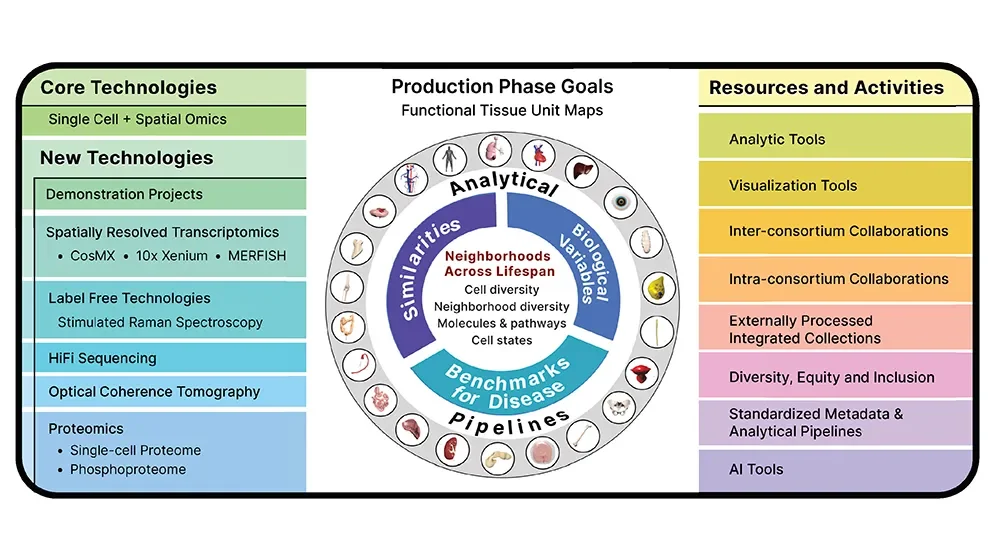
During the process of growth from fertilized egg to complete organism, our cells divide and differentiate, eventually forming specialized tissues and organs. Each is still comprised of cells that perform different and vital functions—neurons in the brain, cardiomyocytes in the heart, hepatocytes in the liver, and so on—but for many decades researchers had to study them at the tissue, not individual cell, level. The recent emergence of single-cell research capabilities has provided the ability to investigate our bodies cell-by-cell, however, and the work has already revealed extensive and sometimes surprising cellular variability, even within organs with highly specific functions. Researchers now also have the ability to map biomolecule locations within individual cells.
Launched in 2018 through the National Institutes of Health Common Fund, the Human BioMolecular Atlas Program (HuBMAP) is building upon these findings to develop spatial maps of non-diseased human organs at single-cell resolution. Given that the number of cells in a human body is estimated to number in the trillions, it’s not surprising that HuBMAP is a massive undertaking that involves more than 400 researchers from over 60 institutions, prominent among them a Jackson Laboratory (JAX) team led by ProfessorPaul Robson, Ph.D. And before any maps could be built, HuBMAP first had to establish the research infrastructure, tools, and standards necessary to coordinate data collection and integration across the many teams involved. “Advances and Perspectives for the Human BioMolecular Atlas Program (HuBMAP),” a paper published in Nature Cell Biology, presents the work done to date to establish the program’s frameworks as well as future steps.
Three-dimensional puzzles
As the term “BioMolecular” implies, each cell included in the project is being extensively characterized. The sample datasets, covering 14 organs across 180 donors, include transcriptomics (all the nuclear and cellular messenger RNA molecules), chromatin accessibility mapping (regions of DNA that are more or less accessible for gene transcription), proteomics (all the proteins within the cell), and much more. The data yields a detailed three-dimensional (3D) molecular picture within single cells as well as defining cell types within 3D maps of tissues and organs. The first phase of the project, which wrapped up in 2022, established standard terminology and anatomical structure reference frameworks for the mapping projects across scales, from the entire human body to organs to functional tissue units (e.g., alveoli in the lung, islets in the pancreas) to cells and, finally, molecular markers within cells.
The program has already yielded significant biological findings. Of particular interest are new insights into cellular relationships within functional tissue units, the identification of rare or previously undocumented cell types and mechanisms, and associations between these previously unrecognized factors with normal function and disease. Moving forward over the next three years, the HuBMAP production phase will focus on building the 3D maps themselves, as well as collecting data from donors representing broad demographic features, such as sex, race/ethnicity, and age. It will also improve analytical and visualization tools as well as integration and interpretation protocols to enhance the tissue atlases of the human body.
The JAX contribution
In addition to Robson, the JAX team working in the HuBMAP program are Director, Single Cell Biology Elise Courtois, Ph.D., Associate Director, Single Cell Biology Bill Flynn, Ph.D., Associate Research Scientist Santhosh Sivajothi, Ph.D., and Postdoctoral Associate Ramalakshmi Ramasamy, Ph.D. They are part of the team mapping the placenta for HuBMAP, and their work complements that for another massive project, SenNet. SenNet is investigating senescent cells—cells that have stopped dividing in response to stressors—in various tissues in humans and mice, also at the single-cell level. JAX is involved with two SenNet tissue mapping centers across both research campuses in Maine and Connecticut, the latter in collaboration with the UConn Center of Aging. Both are profiling the placenta, kidney, and pancreas, as well as human adipose tissue and mouse heart tissue. That project is leveraging the ongoing HuBMAP efforts in the various tissues.
“JAX’s involvement in these huge programs ensures that our technologies remain state-of-the-art and that our Scientific Services can provide JAX researchers with the most advanced capabilities,” says Robson. “The data produced are also of the depth and breadth needed to develop the bridge between human and mice that can increase the relevance of model organism research and accelerate the translation of research discoveries to medical progress.”

