The development and characterization of humanized mice (immuno-deficient mice engrafted with a human immune system) continues to advance. NSG™ mice (005557) genetically modified to express human KITL (Stem Cell Factor; SCF), CSF2 (GM-CSF), and IL-3 (NSG™-SGM3 013062) enhance human hematopoiesis and improve immunity. While IL-3 and CSF2 support human myeloid cell expansion, SCF improves both human hematopoietic stem cell and mast cell expansion. A collaborative study undertaken by groups at Northwestern University, University of Massachusetts Medical School, and The Jackson Laboratory recently examined mast cells in humanized NSG™-SGM3 mice, and demonstrated their maturation and function in vivo. Their findings were reported in the Journal of Allergy and Clinical Immunology (Bryce et al. 2016).
Mature mast cells develop in BLT humanized NSG™-SGM3 mice
NSG™ and NSG™-SGM3 mice were engrafted with human liver, thymus, and hematopoietic stem cells (commonly called BLT-humanized mice) and the percentage of human cells in peripheral blood was evaluated 12 weeks later. The blood from both humanized hosts contained an average of 70% human CD45+ leukocytes, but differed in the percentage of these cells that were from the myeloid lineage: in BLT NSG™ mice 20% of these cells were CD33+ myeloid cells, but in BLT NSG™-SGM3 closer to 60% were CD33+. The blood of BLT NSG™-SGM3 mice contained, on average, about 2% CD117+ CD203c+ human mast cells, whereas these cells were nearly undetectable in BLT NSG™ mice. Cells collected by peritoneal lavage from BLT NSG™-SGM3 also contained significantly higher numbers of human CD33+ myeloid cells and CD117+ CD203c+ mast cells compared similar cell isolates from BLT NSG™ mice. When the functional maturity of the mast cells isolated from the humanized was examined by staining for the marker Siglec-8, BLT NSG™-SGM3 mice had a higher percentage of mature Siglec-8+ cells. Importantly, the Siglec-8+ human mast cells also were positive for FceRI. This receptor binds IgE and when cross-linked by an IgE antibody-antigen complex causes degranulation and release of pro-inflammatory molecules.
The authors also compared the two hosts for differences in mast cell tissue engraftment by histochemical staining for human tryptase, a marker for mast cells. BLT NSG™ mice had detectable tryptase-positive cells in lung and spleen. BLT NSG™-SGM3 mice, on the other hand, had greater numbers of mast cells in these tissues, as well as tryptase-positive cells in small intestine, heart, stomach, and skin. Together, these results demonstrate that BLT-humanized NSG™-SGM3 mice support a higher percentage and higher absolute numbers of mature human mast cells in blood, peritoneum, and peripheral tissues than BLT-humanized NSG™ mice.
Humanized mouse-derived mast cells are functional in vitro
Given the evidence that the human mast cells that develop in BLT NSG™-SGM3 mice have a cell surface phenotype and tissue localization matching that of normal human mast cells, the next important question was whether the cells were functionally active. Mast cells were collected from BLT NSG™-SGM3 mice by peritoneal lavage and were placed in liquid culture. The cells were cultured with stem cell factor, which supported survival and improved cell purity, but did not lead to significant expansion. The cultured cells maintained a phenotype comparable to prospectively isolated primary human mast cells. Next, the cells were incubated overnight with an IgE monoclonal antibody (chIgE-anti-NP) that specifically recognizes a hapten conjugated to bovine serum albumin (NP-BSA). NP-BSA was added to the cultures, and the cells were monitored for degranulation by release of b-hexosaminidase from the lysosomal compartment. NP-BSA stimulated b-hexosaminidase release in a dose dependent manner. Degranulation requires calcium channel activity, so cells sensitized with chIgE-anti-NP followed by NP-BSA activation also were examined for calcium flux. Cells were loaded with fluo-4 NW, a green fluorescent dye that lights up in the presence of calcium, and then challenged with NP-BSA. NP-BSA activation initiated calcium flux, further validating FceRI-mediated activation and degranulation of mast cells through an IgE-allergen complex.
Mast cells also can be activated through non-IgE mediated mechanisms, including components of complement (C3a and C5a) and the neurotransmitter protein Substance P. Cultured peritoneal mast cells from BLT NSG™-SGM3 mice were incubated with each of these molecules independently, and all three stimulated dose-dependent activation and degranulation measured by b-hexosaminidase release and up-regulation of the degranulation marker CD107a. Together, these experiments demonstrated that the human mast cells in BLT NSG™-SGM3 mice were functional through both IgE-dependant and IgE-independent pathways.
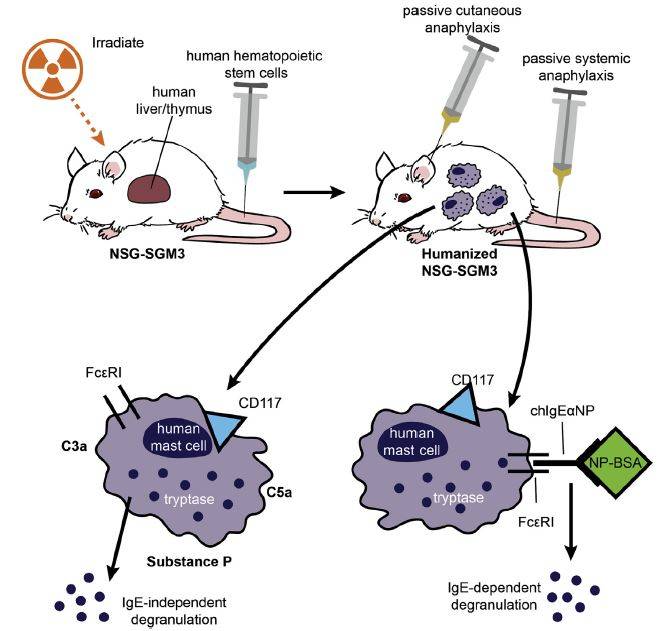
BLT-humanized NSG™-SGM3 mouse-derived mast cells are functional in vivo
Finally, the BLT NSG™ and NSG™-SGM3 mice were compared for in vivo human mast cell functional activity. Mice were treated by intradermal sensitization with either vehicle or chIgE-anti-NP in the ear pinna. One or twenty-four hours later, the mice were injected intravenously with NP-BSA to activate sensitized mast cells. Only chIgE-anti-NP-sensitized ears of BLT NSG™-SGM3 mice exhibited an antigen-stimulated, pro-inflammatory swelling response at both time points. In a separate experiment, BLT NSG™-SGM3 mice were primed systemically by intravenous delivery of chIgE-anti-NP. Twenty-four hours later, the mice were injected intravenously with NP-BSA, and were examined subsequently for response by a drop in body temperature and a physical body scoring system that included scratching, edema, pilar erection, labored breathing, reduced motility and tremors. Only sensitized mice responded to NP-BSA challenge as noted by a decline in body temperature and worse physical body scores across multiple measurements. These results clearly demonstrated the human mast cells in these mice were functionally active as measured by both passive cutaneous and passive systemic anaphylaxis.
The findings published by Bryce et al. are an important leap forward for allergy research. Primary human mast cells are difficult to obtain prospectively. The BLT NSG™-SGM3 strategy provides a unique and reliable approach for generating human mast cells for in vitro analysis, and should enable researchers to uncover more details about the basic biology of these cells. BLT NSG™-SGM3 mice also should be useful as a small animal model for developing preclinical data for allergy-ameliorating therapeutics. We hope that this humanized mouse platform will stimulate the development and testing of human-specific therapeutic agents and accelerate their translation from the preclinical setting to clinical trials and patient treatment.
Reference
Bryce P, et al., 2016. Humanized mouse model of mast cell-mediated passive cutaneous anaphylaxis and passive systemic anaphylaxis. J Allergy Clin Immunol. Apr 6. pii: S0091-6749(16)30008-2. doi: 10.1016/j.jaci.2016.01.049. [Epub ahead of print] PMID: 27139822