Electron microscopy reveals dark microglia and their novel phenotype
March 29, 2016The mysteries of microglia
Microglia have drawn significant attention from researchers recently, owing to their unique role as the resident myeloid cell in the central nervous system. They play critical; although not completely understood roles in brain development and function besides immune surveillance, including neural development, synaptic remodeling, and behavior—functions that differ significantly from the roles of other peripheral macrophages, despite their shared myeloid lineage (Crotti et al., 2016). The origin of these cells has eluded researchers for decades. Current understanding suggests that microglia arise from yolk sac progenitors and maintain themselves throughout life through self-renewal; moreover, despite evidence that monocytes can be recruited into the brain, they do not appear to contribute substantially to maintaining the pool of resident microglia (Ginhoux et al., 2013).
Microglial cells are under investigation as a therapeutic target for treating many neurological conditions, including neurodegenerative diseases, age-related cognitive decline, and stress-related affective disorders like depression. Studies of microglial function across a variety of physiologic states have been complicated by a lack of specific markers to differentiate microglia from monocytes that have been recruited to the brain from the periphery (Greter et al., 2015). A recent collaborative study from Dr. Marie-Ѐve Tremblay’s group atCentre de recherche du CHU de Québec used electron microscopy (EM) to examine ultrastructural characteristics of microglia at synapses in mouse models with neurological conditions (chronic stress and Alzheimer’s disease) that produce highly reactive microglia. They found cells with a distinctive electron-dense “dark” phenotype, a first step towards identifying markers that may accelerate our understanding of these cells in the pathogenesis of multiple brain disorders (Bisht et al., 2016).
“Dark” microglia show distinctive features that set them apart from “normal” microglia, and their numbers increase during stress and degeneration
Models of both stress and neural degeneration were used for the Tremblay study. Chronic unpredictable stress experiments evoked by air puffs, random changes to drinking water access, and forced social interactions were conducted using 12–16-weeks-old mice: wild-type C57BL/6J (Stock# 000664) and B6.129S4-Cxcr3tm1Arsa/SoghJ (Stock# 023337). CXCR3, also called fractalkine receptor, is present almost exclusively on microglia and is a critical regulator of microglial function. Recent work in a complementary study by some of the same investigators showed that disrupting the interaction between CXCR3 and its chemokine ligand, CX3CL1 (mainly produced by neurons) blocks the effects of chronic stress on microglial function (Milior et al, 2015). To examine microglia in a neurodegenerative state, B6C3-Tg(APP695)3Dbo Tg(PSEN1)5Dbo/J mice (Stock# 003378), were used. This model expresses a chimeric amyloid precursor protein (APPSwe) and the human A246E variant of presenilin 1. Both stress and neurodegeneration significantly increased the numbers of “dark” microglia, which showed a darker, more electron dense appearance than either “normal” microglia or circulating monocytes, as shown in Figure 1.
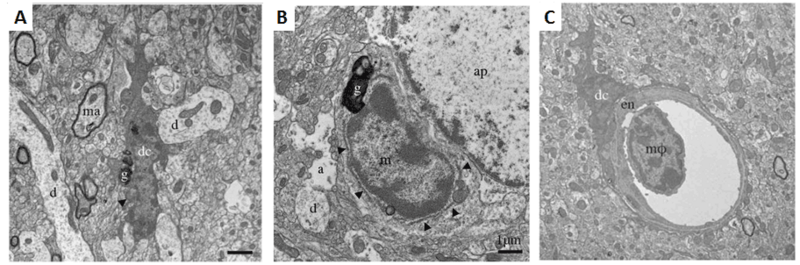
Figure 1. Ultrastructural features differ among brain myeloid cells. A. Example of a dark microglial cell (dc) near a myelinated axon (ma) and multiple dendrites (d) in the median eminence of the hypothalamus of a C57BL/6J control mouse.B. Near an astrocytic process (a) and astrocytic perikaryon (ap), an unstained “normal” microglial cell (m) with less electron dense cytoplasm and nucleoplasm than the dark microglial cell in A. Arrowheads show endoplasmic reticulum. The microglia shown in both A and B contain lipofuscin granules (g). C. A dark microglial cell body (dc) juxtapositioned against endothelium (en) in the CA1 radiatum of an APP-PS1 6-month-old mouse. A bone marrow-derived monocyte or macrophage (mφ) present in the vessel lumen shows a different myeloid ultrastructural phenotype with scant cytoplasm and a chromatin pattern more similar to normal than dark microglia. Scale bars 1μM. (Adapted from Bisht et al., 2016).
Dark microglia cluster together and encircle elements
Dark microglia tended to cluster together and frequently were observed in contact with vasculature. Normal microglia had thicker processes that tended to focally contact other cellular elements, while dark microglia had thinner, more spindle-shaped processes that tended to encircle the elements with which they interacted, such as synaptic processes.Figure 2shows dark microglia clustered around an amyloid plaque in the brain of an Alzheimer’s mouse. Dark microglia were observed also encircling dystrophic neurites. The tendency to enclose other elements suggests that dark microglia have more phagocytic activity than normal microglia.
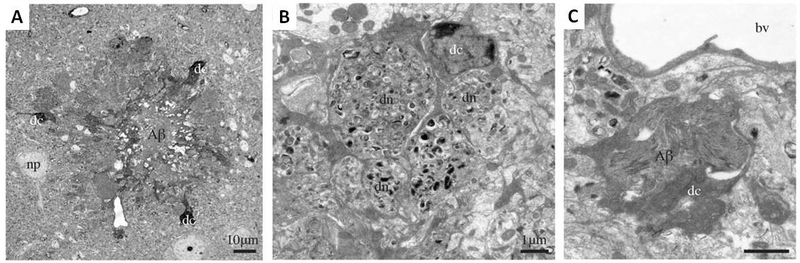
Figure 2. Dark microglia associating with amyloid beta plaques in prefrontal cortex subgranular layers in 6-month-old APP-PS1 mice. A.An amyloid-beta plaque (Aβ) surrounded by three dark microglial cells (dc). A neuronal perikaryon is nearby (np). B.A dark microglial cell with processes encircling dystrophic neurites (dn). C.Near a blood vessel (bv), a dark microglial cell containing a deposit of beta amyloid. Scale bars for A: 10μM; B,C: 1μM. (Adapted from Bisht et al., 2016).
Dark microglia have different immunostaining characteristics, and their presence is not CCR2-dependent
In addition to examining dark microglia via EM, pre-embedding immunostaining was used to determine what markers co-localize with them in unstressed and stressed mice. While normal microglial show strong, diffuse reactivity for Ionized calcium binding adaptor molecule 1 (IBA1), a microglia/macrophage specific calcium binding protein, the signal in dark microglia is faint and punctate. The myeloid cell marker CD11b, a critical component of phagocytic receptor CR3, was strongly expressed in dark microglia at the plasma membrane of processes encircling synaptic components. At the extremities of ramified processes, dark microglia stained positively for 4D4, but not P2RY12, both of which are markers of homeostatic microglia. The origin of these cells also was investigated using CCR2 knockout mice. Previous work had shown that peripheral monocytes infiltrate the brain in a CCR2-dependent manner. Dark microglia cells were found in CCR2-knockout mice, suggesting that they are either of central origin or are recruited to the brain in a CCR2-independent manner.
Electron microscopy still matters
EM technology still enables novel observations not possible through biochemical techniques. Although the accessibility and reduced cost of immunohistochemical markers probably has contributed to fewer published EM-based studies, the technique has a long-standing and successful record in advancing fundamental contributions to cell biology and continues to evolve. Our new age of computational biology and 3D reconstruction may lead to a revival and an important role for ultrastructural analysis in future discoveries (Afzelius et al., 2013).
Summary
Although dark microglia have not yet been reported in human brains, these data in mice suggest that these cells increase in the brain during pathological states. Although dark microglial are only recognizable currently using electron microscopy, this study provides a starting point for identifying immune-markers that will be useful to investigators that may not have access to ultrastructural techniques. Such steps are essential in developing much-needed tools for better understanding the role of microglia in the pathogenesis of multiple brain disorders.
