Your quarterly update from the MCGI team April 2023 — June 2023
Your quarterly update from the MCGI team April 2023 — June 2023
MCGI is developing educational materials and resources for Maine cancer patients about cancer biomarker testing and personalized medicine.
We are in search of volunteers who are open to sharing their healthcare experiences involving genomic and genetic testing. We are seeking advisors who can offer a patient’s perspective and assist us in developing user-friendly forms and educational materials that can be easily understood and utilized by all patients and their families. Furthermore, we are interested in exploring new avenues to ensure patients have access to these valuable resources.
Why become a patient or family advisor?
What are the responsibilities of a patient or family advisor?
How to get involved?
If you are a cancer survivor, a caregiver, or a family member who has experienced the impact of cancer firsthand, we invite you to join our team as a patient or family advisor.
For more information, including how to volunteer, contact Sheila at 860-837-2326 (M-F 8:00am-4:30pm) or email mcgi@jax.org.
Visit our website for more information on the MCGI Patient Advisory Committee.

The Maine Cancer Genomics Initiative: Implementing a Community Cancer Genomics Program Across an Entire Rural State highlights the successful integration of clinical genomics and precision oncology throughout the entire rural state of Maine, achieved through the collaborative efforts of the initiative and the local oncology community.
In addition, The Jackson Laboratory's Mark Wanner reflects on this recent publication in his latest featured article.
 George is 73 years of age and has low-risk, localized prostate cancer. You explain to George and his adult daughter Maureen, that you believe active surveillance is the best course of action, but surgery or radiation are also options. Maureen mentions that a family friend with cancer has had a large panel biomarker test. She asks whether a similar test will be used to determine additional treatment options for her father.
George is 73 years of age and has low-risk, localized prostate cancer. You explain to George and his adult daughter Maureen, that you believe active surveillance is the best course of action, but surgery or radiation are also options. Maureen mentions that a family friend with cancer has had a large panel biomarker test. She asks whether a similar test will be used to determine additional treatment options for her father.How do you explain to George and his daughter why a large panel biomarker test is not recommended at this time?
Find the answer to this question and practice with more cases in our newly updated module Exploring Cancer Biomarker Testing. You can earn free CME and CNE!
The 2023 Forum drew in 246 in-person and virtual attendees for sessions that reflect MCGI’s next steps, termed MCGI 2.0. Now that precision care is being offered in every Maine oncology practice, the presentations and discussions covered a wide variety of topics and concerns about how MCGI can be made even more effective. How can the remaining barriers to care among the state’s most rural and/or low-income patients be addressed? How can cancer patients be better engaged and how can their insights help improve overall patient experiences once in the clinic? What technologies, such as artificial intelligence, have the most potential to change cancer research and clinical care? How can MCGI’s current offerings, including genomic tumor boards, access to clinical trials, implementation of the latest research findings, and more be further enhanced? The Forum made clear that, as exciting as the last six years have been, MCGI’s future impact on cancer care, both in Maine and far afield, is likely to be even more profound.
Session 1: MCGI Updates
Session Chair: | Leah Graham, Ph.D., Associate Director, MCGI, The Jackson Laboratory | |
Speakers:
| Sarah Sinclair, D.O., Medical Director of Hematology & Oncology, Director of Clinical Research, Northern Light Cancer Care Eric Anderson, Ph.D., Faculty Scientist, MaineHealth Institute for Research | |
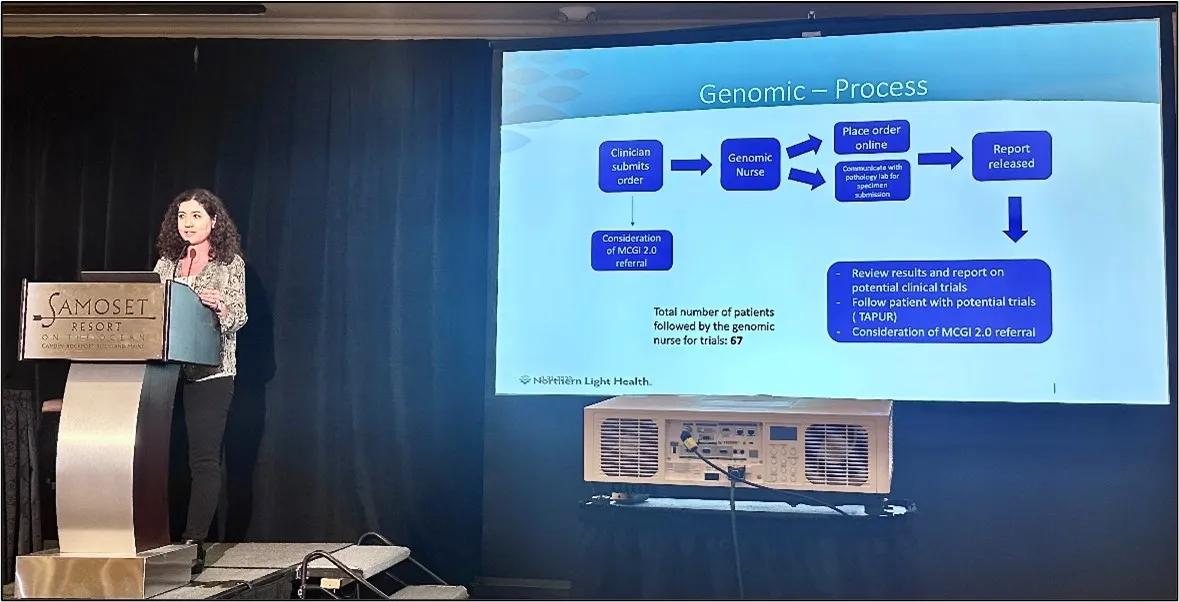
The MCGI update session provided two different perspectives: what care is being delivered within a specific healthcare system, and what are patient expectations for, knowledge of, and attitudes toward the care delivered throughout MCGI? Sarah Sinclair noted that Northern Light Cancer Care, based in Brewer, Maine, is highly active with MCGI and serves a mostly rural patient population. Northern Light has a large research team on staff, has ordered 275 genomic tests over the past nine months alone, and currently has 53 clinical studies available to patients across multiple disease types. It is also participating in the national TAPUR clinical trial detailed in a subsequent talk, which JAX coordinates. Eric Anderson presented about patient perceptions of genomic tumor testing and outcomes in MCGI, including how rurality and socioeconomic factors can influence them. MCGI’s patient cohort is unusual, in that it is lower income and less urban than most precision oncology patient groups. The findings from the MCGI 1.0 data gathered in 2017-2020 were published just this month.

Session 2: Patient Experience and Advocacy
Session Chair: | David Thomas, FRACP, Ph.D., Head, Genomic Cancer Medicine, Omico: Australian Genomic Cancer Medicine Centre | |
Speakers:
| Meghna Trivedi, M.D., M.S., Assistant Professor, Columbia University Irving Medical Center Barbara Segarra-Vázquez, D.H.Sc., Professor and Principal Investigator at CRECD NIH Grant, University of Puerto Rico | |
The pursuit of therapies that are toxic to cancer cells can yield treatments that are also toxic for patients, physically and financially. Attention is now being focused on patient experience in the context of targeted therapies. Meghna Trivedi explored the important differences between physician assessments of toxicities and patient-reported outcomes, the latter of which yield more insight into side effects and other therapy impacts. She also discussed the determination of maximum tolerated doses (MTD) during clinical trials and the need to incorporate more data into dose selection, as many MTDs have had to be changed because of subsequent side effect issues after FDA approval. Researcher and cancer patient Barbara Segarra-Vazquez provided a patient perspective for cancer treatment, advocating for shared decision making between health care providers and patients to help determine what is actually best for the patient. There are barriers, including disease and treatment complexity, time pressures, health literacy and more, especially with the advent of targeted therapies based on molecular data. But patients need to understand how the therapies work and know the options available to them.

Session 3: The Future of Molecular Testing in Treatment Decisions
Session Chair: | Lindsey Kelley, M.P.H., M.S., CGC, Genomic Navigator, MCGI, The Jackson Laboratory | |
Speakers:
| Stacy Gray, M.D., A.M., Deputy Director, Center for Precision Medicine and Division Chief, Clinical Cancer Genomics, City of Hope Christopher Gocke, M.D., Associate Professor of Pathology, Oncology, and Genetic Medicine, Johns Hopkins University School of Medicine | |
Molecular testing and associated technologies now play important roles in oncology. The use of advanced tools to improve cancer care can therefore be applied in very different contexts. Stacy Gray addressed the difficulty to communicating about genomic information and the resulting underutilization of genomic testing and targeted therapies, particularly in underrepresented minority groups. To address the problem, City of Hope developed HOPE-Genomics, a patient-facing app that displays personalized genomic testing results. Based on an early prototype and subsequent feedback, they are developing the app to be as user-friendly as possible and have an NHGRI grant to thoroughly test its effectiveness. Christopher Gocke presented on a different part of cancer care technology, the dynamic analysis of genomic test results using a cloud-based AI platform that accesses the latest information from clinical trials, publications and genomic databases for analyses. A serial re-analysis of more than 2,000 patient records over a nine-month period revealed that the percentage of patients who had a targeted therapy for their tumor profile available increased from 11%->21%, indicating AI could be used to make analysis a dynamic process over time.

Session 4: Insights into the Genetics of Ovarian Cancer and Oncofertility
Session Chair: | Leslie Bradford, M.D., Gynecologic Oncologist, Maine Health | |
Speakers: | Ewelina Bolcun-Filas, Ph.D., Associate Professor, The Jackson Laboratory
Charlie Gourley, Ph.D., Professor and Honorary Consultant, Medical Oncology, Clinical Director, Cancer Research UK Scotland Centre, Director, Nicola Murray Centre for Ovarian Cancer Research, The University of Edinburgh | |
It is essential to better understand cancers and associated therapies that affect individuals with uteruses and younger women who may later wish to have children. Side effects for standard chemotherapy and radiation include infertility, sexual dysfunction and premature menopause, which increases the risks of osteoporosis and other age-related diseases. Ewelina Bolcun-Filas uses mice and in vitro organoids to study oocyte function and therapy toxicity, with the goal of finding ways to preserve follicular function and fertility. Her research has helped reveal specific mechanisms that eliminate oocytes following irradiation, with potential therapeutic targets. The belt between Edinburgh and Glasgow in Scotland has a high incidence of cancer, and Charlie Gourley focused on the molecular traits of ovarian cancer itself based on his work there. He discussed the roles of chromothripsis (severe genomic disruption) and ecDNA in ovarian cancer initiation and progression, as well as the important clinical differences between high and low grade serous ovarian cancers. Research continues into the factors that contribute to variable precision treatment responses and therapy resistance mechanisms.

Session 5: New Developments in Translational Research
Session Chair: | Edison Liu, M.D., Professor and President Emeritus, The Jackson Laboratory | |
Speakers: | Roel Verhaak, Ph.D., M.Sc., Professor of Neurosurgery, Yale School of Medicine
Jeffery Chuang, Ph.D., Professor, The Jackson Laboratory | |
Clinical progress is based on research discovery, in a process that has traditionally taken many years, if not decades, to proceed from bench to patient. Today, new research capabilities are accelerating the translation process in oncology and all other medical areas. Roel Verhaak presented his research into a cancer that remains among the most difficult to effectively treat: glioma, a malignant brain cancer that inevitably recurs. He has spearheaded the GLASS Consortium, a collaborative program that analyzes gliomas pre- and post-treatment to reveal the impact of standard-of-care treatments—surgery, chemotherapy and radiation—on tumor characteristics, including mutations, epigenetic changes, and more. Jeffery Chuang is working to implement machine learning-based imaging analysis to computationally generate a clinically actionable description from cancer sample imagery. Tumor types can already be clearly distinguished from images by neural networks with high accuracy. The current focus is to see cancer in many more colors than the current staining regimens provide to reveal far more complexity within the tumor samples, such as individual proteins and RNAs.

Session 6: New Developments in Clinical Care
Session Chair: | Christian Thomas, M.D., FASCO, Director, Clinical Research, New England Cancer Specialists | |
Speakers: | David Thomas, FRACP, Ph.D., Head, Genomic Cancer Medicine, Omico: Australian Genomic Cancer Medicine Centre Ryan Sullivan, M.D., Associate Professor, Massachusetts General Hospital | |
As genetic and genomic testing and analysis of tumors becomes more common, patient stratification and the application of targeted therapies is getting ever more refined. But significant challenges remain, and a large number of tested patients are still not able to access an approved therapy that targets their tumor profile. Indeed, at this time 42% of all cancer deaths are due to rare or less common cancers, and David Thomas is working to address this unmet need. Omico, a non-profit national precision oncology network in Australia, has now united the eight states and territories to work together for the purpose. The innovative model for research-led cancer care has been successful, and there are now 23 centers throughout the country. Ryan Sullivan presented on novel approaches to MAP kinase inhibitors and RAS targeting, and the very complicated molecular systems involved. KRAS is the most commonly mutated oncogene in cancer (~20%), but it has been known as “undruggable.” Inhibiting related pathways such as RAF, MEK and ERK has been explored, so far without success, but recent work has revealed the potential for drugs that convert RAS to its inactive state.

Session 7: Clinical Trials for Rural Healthcare
Session Chair: | Vatche Tchekmedyian, M.D., Hematology/Medical Oncologist, MaineHealth | |
Speakers: | Owen Hahn, M.D., Associate Professor, Medicine, The University of Chicago Richard Schilsky, M.D., FACP, FSCT, FASCO, Principal Investigator, ASCO TAPUR Study, American Society of Clinical Oncology Christine Lu-Emerson, M.D., Neuro-Oncologist, MaineHealth | |
The final session returned to the challenges of oncology in low-population areas such as Maine and how to expand rural patient access to clinical trials. Olwen Hahn noted the higher mortality and slower decline in death rates for rural compared to urban patients. Overall, only 3-8% of all cancer patients enroll in clinical trials, and rural, elderly and diverse populations are relatively underrepresented. The low participation is driven by availability, including travel logistics, and communication, as research has shown that more than half of patients would participate if they could and were actually asked. Decentralized trial approaches combined with patient concierge services, including help with transportation, childcare and more, could provide more rural friendly clinical trials and increase participation rates. Richard Schilsky presented on the ASCO TAPUR trial, which just passed its seventh anniversary and is now conducted at 252 sites in 28 states. TAPUR is described as a pragmatic study to learn from precision medicine in practice, with a single master protocol, single IRB, broad eligibility, extensive physician discretion and no auditing. About 90 genomic alterations in any solid tumor are applicable, and more than 3,800 patients have been registered, with more than 2,600 enrolled. TAPUR’s ongoing priorities are rapid dissemination of results, adding new drugs and combinations, adding new sites, and sharing data across complimentary international studies. The final presentation by Christine Lu-Emerson covered comprehensive genomic tumor testing and the usefulness of genomic tumor boards for brain tumors. Fast, accurate diagnoses are important for prognostic and therapeutic purposes, and routine incorporation of comprehensive molecular profiling and genomic tumor board assessment into the initial diagnostic workup may improve patient experience. A study is being launched to assess the response of participants, process feasibility and timelines to see if it is indeed a better and faster process or if it introduces unnecessary complications and delays.

Genomic Tumor Board Case Discussions
Session Chairs: | Emily Edelman, M.S., CGC, Director, Clinical Education Program, The Jackson Laboratory Kate Reed, M.P.H., Sc.M., CGC, Director, Precision Oncology Education, Clinical Education Program, The Jackson Laboratory | |
Speakers:
| Jill M. Kolesar, Pharm.D., M.S., BCPS, FCCP, Professor, Pharmacy and Medicine, University of Kentucky Christine Walko, Pharm.D., BCOP, FCCP, Senior Member, Individualized Cancer Management, Moffitt Cancer Center Kelsey Fusco, M.S., CGC, Genetic Counselor, New England Cancer Specialists Sara Patterson, Ph.D., Associate Director, Clinical Genomics Informatics Products, The Jackson Laboratory Cara Statz, Ph.D., Senior Clinical Analyst, The Jackson Laboratory | |
The MCGI’s Genomic Tumor Board (GTB) now serves as a model for other precision oncology efforts. But what, exactly, does it do? To give Forum participants an in-depth look, both days featured guided discussions of representative cases of the kind covered by MCGI’s GTB. Specific clinical and test result data was provided and options discussed regarding indicated therapies for one patient each day. The discussions revealed many of the issues each case can raise for the GTB and how the data, while vital for informing treatment, does not necessarily provide clear answers for the oncologist. The sessions emphasized the importance of expert consultation and clear communication to provide both oncologist and patient with the information needed to make informed decisions regarding the best path forward for the patient.
Written by Mark Wanner
Connect with us online and be part of the MCGI community!