By Jens Rueter, M.D. and Andrey Antov, Ph.D., M.B.A.
September was a banner month for the Maine Cancer Genomics Initiative! Another 5 practices have on-boarded with the Study, enabling them to enroll patient participants. In addition, 4 sessions of the MCGI Genomic Tumor Board (GTB) have been held. The MCGI-GTB assists clinicians with test results interpretation and leverages the expertise of the larger oncology community. In a network-building spirit, these sessions have been hosted at three different practices as well as at JAX.
In order to enable the 9 practices currently participating in the study to enroll we have executed 25 separate agreements. These agreements span study site agreements, letters of support, and agreements with independent and affiliated pathology groups.
To learn more about MCGI, please visit our recently updated website at www.jax.org/mcgi, now including biographies of Clinical Steering Committee members and our external advisors. The Initiative is thankful to our External Advisors for sharing their expertise in Genomic Tumor Board sessions. We are also thankful for the ongoing guidance and expertise of our Steering Committee members. In each newsletter we will feature a Steering Committee member in this Spotlight.
This month we feature Steering Committee member Dr. Elizabeth (Betsy) Connelly. Dr. Connelly leads the MCGI study efforts as the Principal Investigator for Waldo County General Hospital and Pen Bay Medical center.

Elizabeth (Betsy) Connelly, D.O.
Dr. Connelly practices medical oncology and hematology at Waldo County General Hospital and Pen Bay Medical Center. She is board certified in Medical Oncology, Hematology, and Internal Medicine. Dr. Connelly is a member in the American Society of Clinical Oncology and is on active staff at Waldo County and Pen Bay Medical Center. She received her DO from Texas College of Osteopathic Medicine followed by a residency at Akron General Medical Center in Internal Medicine and a Fellowship with the Cleveland Clinic Foundation in Medical Oncology/Hematology.
By Petra Helbig, CCRP
The MCGI study protocol was amended to incorporate input from participating sites and locations. Version 2 of the protocol and informed consent form were recently distributed to the sites by WIRB. Please be sure you are using the most recent version of the consent form and other study documents.
In total the MCGI study is currently open at 9 practices, allowing more than 20 enrolled clinicians to enroll patient participants as well. We are happy to assist you with the process or answer any questions you may have.
If you are interested in more information, please reach out to us at [email protected].
By Andrew Hesse, Clinical Data Analytics & Reporting Manager
The JAX Action-Seq™ report contains a wealth of information about a patient's tumor testing results. Over the course of several newsletters, we will highlight different aspects of the report and provide details that can help in interpreting the information provided.
In this issue we present: CLIA Educational Brief - Joint AMP/ASCO/CAP Variant Classification.The Clinical laboratory at JAX is one of the earliest adopters of the recent 2017 joint (AMP/ASCO/CAP) guidelines on interpretation and reporting of somatic variants in cancer1. The classification system is broken down into 4 tiers (Tier I, II, III and IV; Figure 1).
Figure 1: Stratification of evidence in variant classification
Overview of the types of evidence and their contribution to the different variant classes. Preclinical can enrich support for a variant or justify class upgrade, but will not alone reach Tier II. FDA drug for patient tumor/variant profile is default Tier I. Tier IV variants (not displayed) are benign/likely benign and not included on the final report.
Each Tier contains 3 subclasses (Figure 2): Therapeutic (Rx), prognostic (Px) and diagnostic (Dx).
Figure 2: Examples of Tier I/II Classification
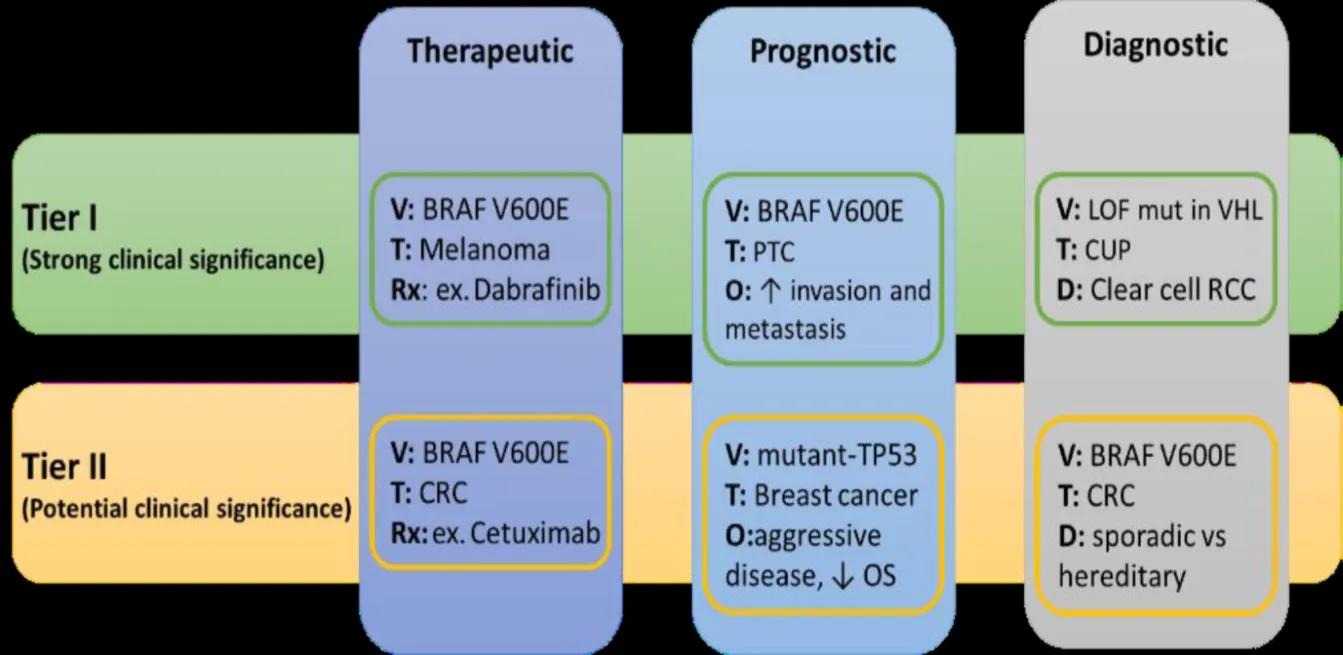
Legend: V= variant, T= tumor type, Rx= Drug, O= associated Px outcome, D= associated Dx outcome, ↑/↓=increase/decrease
Variants in Tier I (strong clinical significance) serve as predictive markers for response to FDA-approved drugs indicated for the patient or recommended by guidelines for testing as Px or Dx markers of the patient tumor. Variants in Tier II (potential clinical significance) serve as markers for either off-label use of FDA-approved drugs or investigational drugs with clinical trial evidence supporting use in the patient tumor or serve as eligibility criteria for inclusion in clinical trials. Tier III (unknown clinical significance) are variants that do not meet the criteria for inclusion in Tiers I, II and IV, or have evidence with conflicting interpretations. Tier IV are considered benign variants and not included in reports as standard protocol. These variants are observed at polymorphic frequencies in large cohorts or have direct evidence demonstrating lack of disease involvement.
1. Li MM, et al. Standards and Guidelines for the Interpretation and Reporting of Sequence Variants in Cancer: A Joint Consensus Recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J Mol Diagn 2017;19:4-23.
As always; Information about test processing status is available by contacting Shannon Rowe in Clinical Lab Customer Service,[email protected]. Shannon's direct line is 860-837-2162. If you have any additional questions related to technical or scientific aspects of the test, please do not hesitate to reach out to our Clinical Laboratory Director, Honey.
By Kate Reed, MPH ScM, CGC
Providing educational opportunities for clinicians enrolled in MCGI is an important component of the Initiative. JAX will be providing two types of education:
Interpreting Somatic Cancer Panel Testing Reports is a self-directed module, which uses interactive cases to explore and apply results from large somatic cancer panel tests. You can earn 0.5 CME credits.
https://learn.education.jax.org/browse/hpe/cme/mcgi
Don't forget about these additional educational opportunities:
Exploring Somatic Cancer Panel Testing is a self-directed module, which uses interactive cases to explore the benefits and limitations of large panel testing for different types of patients. You can earn 0.5 CME credits.
Sept 9th: We had a wonderful response to information offered at our table at the Harold Alfond Center for Cancer Care's Cancer Survivor Day. This community event gave us the chance to interact directly with cancer patients and their family members.
Sept 18th: Andrey Antov, PhD, MBA spoke at the Cancer Registrars Association of Maine meeting to share the message of the MCGI Study and support our educational mission.
Sept 22nd: Jens Rueter, MD gave the keynote speech at the Dempsey Center for the Cancer Genetics Management in the Primary Care Setting workshop.
Oct 5th - 7th: Jens Rueter, MD and Andrey Antov, PhD, MBA attended the Oncology Precision Network (OPeN) conference. This conference focuses on making precision medicine part of routine clinical care.
Oct 7th: Petra Helbig, CCRP and Jennifer Bourne, MS spoke at the Southern Maine Oncology Nursing Society Conference about Integrating Cancer Genomic Testing in Clinical Practice through MCGI.
Oct 27th: Jens Rueter, MD and Andrey Antov, PhD, MBA will attend the Northern New England Clinical Oncology Society (NNECOS) 2017 Annual Meeting. Andrey will present an MCGI abstract and poster at this event.
_____________________________________________________________________________________________________________
The mission of the Maine Cancer Genomics Initiative (MCGI) is to enable widespread access to clinical cancer genomic tests for the Maine oncology community and to increase the understanding of cancer genomics by Maine oncology clinicians. The MCGI, enabled through the generous financial support from The Harold Alfond® Foundation, leverages the strengths of key medical and bioscience research institutions in Maine to create and alliance focused on precision cancer diagnostics and treatment.
The MCGI central office team is dedicated to ensuring your practice's engagement and experience with genomic cancer testing. Please feel free to reach out with any questions or comments. Email us at [email protected].