Battling intracellular superbugs with antibody-antibiotic payload
Blog Post | November 24, 2015Epidemiological Public Enemy No. 1
Commensal bacteria, such as Staphylococcus aureus, commonly populate skin and nasal passages, but can cause minor infections upon entering open wounds. Typically, S. aureus proliferates in the extracellular space and is readily eliminated by β-lactam antibiotics such as methicillin, a penicillin-class drug. Through natural selection and the widespread use of antibiotics, however, methicillin-resistant S. aureus (MRSA) strains represent a significant health concern worldwide. Worryingly, MRSA superbug strains are even becoming resistant to “last resort” medications such as vancomycin, and treatments to destroy them may require more creative, alternative strategies. In a recent issue of Nature, researchers at Genentech in South San Francisco employed antibody-antibiotic conjugates (AAC) to target intracellular reservoirs ofS. aureus, whichmay be MRSA’s Achilles heel in its ability to escape antibiotic eradication. (Lehar et al., 2015).
Immune cells harbor intracellular bacterial fugitives and contribute to drug resistance
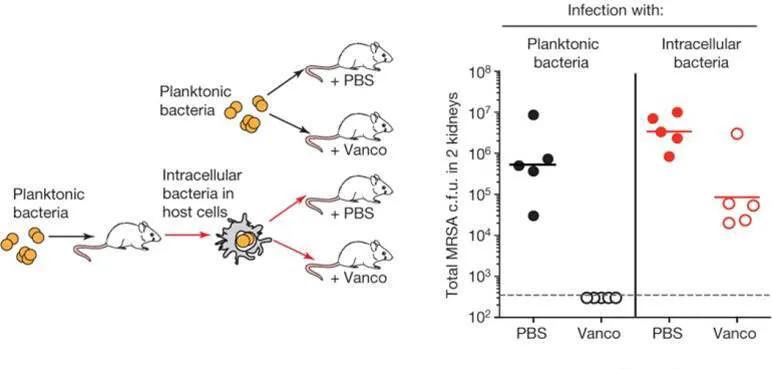
The Genentech group first determined whether intracellular S. aureus were resistant to antibiotics commonly employed against MRSA strains. In a classic minimum inhibitory concentration assay, a highly virulent strain of MRSA that had been engulfed by macrophages could not be killed at clinically relevant antibiotic concentrations that readily killed free MRSA. To determine relevance in vivo, A/J (000646) mice were infected either by intraperitoneal injection with free MRSA or by tail vein injection with MRSA-infected macrophages. Treating the infected mice with vancomycin eliminated MRSA from mice infected with the free bacteria, but MRSA residing within macrophages were able to survive and colonize both the kidney and the brain. Under similar conditionsin vitro, a significant number of bacteria harbored within macrophages also survived treatment. These results suggest that although the majority of macrophage-engulfed MRSA cells might be killed, enough bacteria persist, and may serve as a reservoir to infect other tissues at a later time.
Antibody-antibiotic conjugates infiltrate and eliminate intracellular Staphylococcus aureus
Because intracellular MRSA remain a potential source of continued infection, the Genentech group devised an antibody-drug conjugate, a strategy common in cancer therapies, to target and eliminate these protected bacteria. Antibodies isolated from B cells of human patients that had recovered fromS. aureus infections served as the source for selecting an antibody that could bind a variety of clinically relevant S. aureus strains. The selected S. aureus antibody was then linked to a highly potent antibiotic that would be activated by and could function within the acidic environment of a phagocytic lysosome but could not function extracellularly. This new AAC was tested for its ability to target intracellular MRSA in vivo. As before, MRSA within macrophages were protected from vancomycin treatment; however, a single dose of AAC in the presence of vancomycin prevented the spread of infection to the brain (Fig. 1). In addition, mice intravenously infected with free MRSA and treated 24 hours later with AAC had significantly less infection in their kidneys than those treated with vancomycin at the same time point.
These results indicate that an antibody-antibiotic conjugate that is only activated after entering an infected phagocytic cell is more efficient at eliminating a bacterial superstrain than standard antibiotics that act extracellularly. In addition, these data suggest that bacteria that survive within host cells contribute significantly to infectious disease pathology, and should be considered a potential source of drug resistance.