Targeting tryptophan metabolism exhibits the potential to extend lifespan
Research Highlight | January 16, 2024
New study demonstrates potential life-extending therapeutic targets in the tryptophan-kynurenine metabolic pathway.
An essential amino acid
A research team led by Assistant Professor George Sutphin, Ph.D., of the University of Arizona and Associate ProfessorRon Korstanje, Ph.D., of The Jackson Laboratory has revealed a new role for tryptophan metabolism in aging. Tryptophan is an essential amino acid that our cells use to produce proteins or is a precursor for important hormones, such as melatonin or serotonin. The majority of the tryptophan consumed is metabolized through the kynurenine pathway, which produces many bioactive metabolites, including the crucial coenzyme in energy metabolism, nicotinamide adenine dinucleotide (NAD+). Tryptophan metabolism through the kynurenine pathway is activated in response to inflammation and can in turn regulate immune signaling, oxidative stress and energy production. Dysregulation of this pathway is linked to various age-related conditions such as chronic inflammation, atherosclerosis, neurodegeneration and cancer.
A pro-longevity metabolite and its pathway
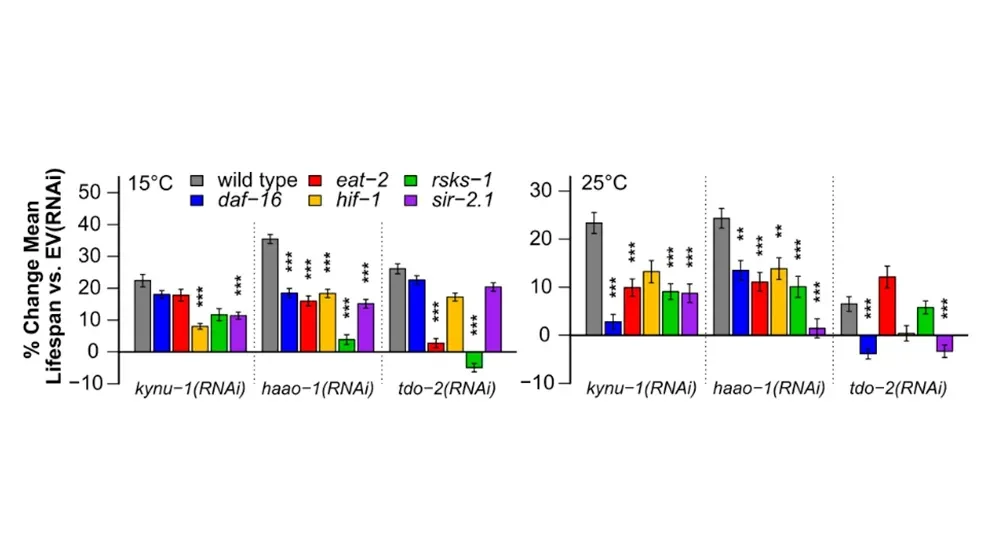
Multiple kynurenine pathway interventions have been previously shown to benefit aging in invertebrates. In “On the benefits of tryptophan metabolite 3-hydroxyanthranilic acid inCaenorhabditis elegans and mouse aging,” a paper recently published in Nature Communications, Sutphin, previously a postdoctoral associate in Korstanje’s lab and a JAX Scholar in Aging, Korstanje, and a team of researchers focused on the enzyme 3-hydroxyanthranilic acid (3HAA) dioxygenase (HAAO). HAAO breaks down a key lifespan mediator in the kynurenine pathway, 3-hydroxyanthranilic acid (3HAA), an intermediate metabolite in the tryptophan metabolic process. Reducing HAAO with a drug or inhibiting expression of its encoding gene raised 3HAA levels in the roundworm,Caenorhabditis elegans, and in mice.
Two potential life-extending therapeutic targets
The research team discovered that increasing 3HAA levels by either inhibiting HAAO or directly feeding animals 3HAA increased healthy longevity in bothC. elegans and mice. They observed an approximately 30% extended lifespan and a delay in age-related health decline in C. elegans, thanks in part to activation of the oxidative stress response. A follow up study in male mice showed that 3HAA-supplemented diets increased lifespan relative to mice on a control diet. The researchers also knocked out theHaao gene in male and female mice, which significantly increased lifespan, though the effect was greater in the female mice. The findings in C. elegans and mice suggest that both HAAO and 3HAA are potential therapeutic targets for promoting healthy longevity, addressing age-related inflammation and mitigating oxidative stress-induced damage. Further study is needed, however, as inhibiting HAAO may potentially delay early-life growth milestones in both worms and mice, negatively affecting reproductive potential. Proper dosing of 3HAA or drugs that inhibit HAAO would also need to be prioritized in future studies, as mutagenic and carcinogenic properties have been observed at higher concentrations.

