MCGI: Bringing precision cancer care to Maine patients
Featured Article | June 13, 2023
When people think of Maine, what usually comes to mind is its natural bounty. It has lobster and blueberries, beautiful rocky coastlines and wild woodlands, and boundless opportunities for outdoor recreation of all kinds. What Maine doesn’t have a lot of, however, is year-round residents. Overall, Maine’s population density is the lowest of any state east of the Mississippi, and given that about 40% of the population lives in the Greater Portland metropolitan area, the remainder of the state is very rural indeed. And while that provides visitors with gorgeous scenery and the chance to escape their urban confines, it leads to areas of sparse infrastructure for many Mainers in their day-to-day lives.
Maine is also the home base for The Jackson Laboratory (JAX), which has been researching cancer and other diseases for more than 90 years on its Bar Harbor campus. For the past 40 years, JAX has been designated as abasic research Cancer Center by the National Cancer Institute (NCI) for its research into the molecular underpinnings of cancer initiation, progression and spread. The research has rapidly expanded our understanding of cancer and has helped lead to significant advances in oncology, including therapies that expose cancers to immune system attack and others that target specific components of cancer growth and survival. But as the new, more precise therapies started moving through clinical trials and FDA approvals a decade or so ago, a sad reality emerged: most Maine cancer patients in JAX’s own back yard lacked access to them.
JAX had a response, and then-President and CEO Edison Liu, M.D., spearheaded the establishment of the Maine Cancer Genomics Initiative (MCGI) in 2016, supported by funding from the Harold Alfond Foundation. A recent paper in JCO Precision Oncology documents the formation and initial efforts of MCGI, whose basic goal was and is to provide all Maine cancer patients with the opportunity to benefit from the latest genomic testing, expert evaluation, and modern cancer therapies. MCGI has many components, and a vital part of the program remains physician and patient education, as the research behind the genomic testing and assessment is progressing quickly, and new cancer therapies and combination strategies are emerging or being sidelined at a rapid pace. The data and analyses presented in the paper are from MCGI’s first stage from 2016-2020, but its expansion and wider scope of contributions in 2023 are indication of its striking success under the leadership of JAX Chief Medical OfficerJens Rueter, M.D., himself an oncologist. Indeed, in its short history, MCGI has profoundly changed the delivery of cancer care in Maine.

Engaging oncologists and patients
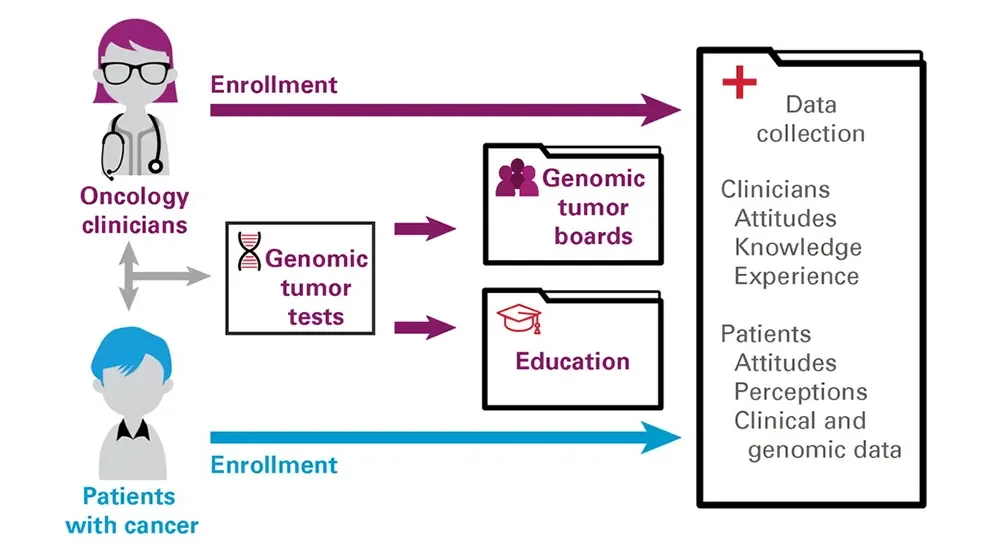
JAX is a biomedical research laboratory with no direct clinical presence in Maine or elsewhere, so the first task facing the MCGI was to engage with the practicing oncologists who work directly with cancer patients. The perception of JAX as a prestigious biomedical research institution helped establish it as a trusted coordinating hub and partner. Over an 18-month period in 2017-2018, MCGI was able to enroll every Maine oncology practice, and by September 2020 a total of 79 physicians, 100% of those eligible, had enrolled in the study. To further support clinical implementation, MCGI offered educational resources to increase their knowledge of and confidence with interpreting results from the genomic tumor tests they ordered. Interest from nonphysician staff led to the development of additional online resources specifically for oncology nurses and research coordinators. MCGI also established an annual two-day CME-accredited forum to provide educational as well as networking and team building opportunities.
Because of the oncologists’ support and commitment, patient enrollment in MCGI also increased rapidly, from 15 patients in 2017 to 162 in late 2018 to 1,610 by October 2020. Underscoring the importance of the MCGI mission, 73% of the patient participants lived in rural areas, and the highest per capita enrollment was from regions with low population densities. A key part of the MCGI program was free, large-panel genomic tumor testing for all enrolled patients, and while some early challenges were encountered with sample collection and specimen requirements for testing, the process improved significantly over time. In the end, 1,292 patients had completed successful testing and received a test report.

Genomic Tumor Boards
Genomic tumor testing is a key component of precision oncology, but interpreting the report results and their implications for patient care can be quite difficult. The precision oncology field has evolved rapidly, and keeping current with cancer genomics, targeted therapies, and clinical impact is a specialty in itself. MCGI therefore established genomic tumor boards (GTBs) comprised of national and international precision oncology experts and JAX laboratory testing and cancer data specialists. The GTBs provided Maine oncologists with direct access to colleagues from the leading academic precision oncology programs for case discussions and treatment decision support.
Each GTB session was organized and moderated by an MCGI team member and had an average of 18 attendees, 36% of whom were oncologists and 26% oncology nurses and other staff members. Interestingly, the transition to fully remote sessions during the COVID-19 pandemic increased both attendance and number of cases discussed per session. The cases presented were selected either by the treating oncologist or MCGI leadership based on potential clinical impact or educational value of the case. In all, 515 patient cases were presented at 196 GTB sessions between August 2017 and June 2021. In the paper, the authors state that GTBs were the most impactful intervention in supporting the interpretation and appropriate use of genomic tumor testing, and clinical participants expressed both strong interest in them and stated that they were beneficial to their practice. The MCGI’s GTB effort is now providing the basis for abroad new study assessing GTBs and whether they ultimately improve cancer patient outcomes.
MCGI 2.0
While the paper only presents data from MCGI’s first phase, the program is forging ahead with renewed Harold Alfond Foundation funding. Called MCGI 2.0, it is addressing additional patient problems and challenges encountered during the launch phase. Of particular note is access to cancer drug clinical trials based on the genomic tumor testing results. It previously required travel to major academic medical centers, and oncologists communicated that the logistics made it difficult if not impossible for many of their rural patients to participate. MCGI has now helped bring precision oncology clinical trials into Maine through the NCI-MATCH and TAPUR studies, an important initial step for increasing patient access. MCGI has also added a central clinical genomic navigator position to further assist patients and oncologists throughout the testing, analysis, and therapy delivery process. MCGI is now serving as a model for cancer care delivery within the U.S. as well as internationally, and its benefits are likely to help cancer patients far beyond Maine’s borders in the years ahead.

