Diabetic B6 mice humanized with stem cell derived beta cells
March 15, 2016In autoimmune type 1 diabetes (T1D), a patient’s own T cells destroy the insulin-producing β cells in the pancreas, leading to progressively worse hyperglycemia. The standard of care for T1D patients is blood glucose monitoring and multiple insulin injections per day. Even with close glucose monitoring, this strategy does not restore physiologic glycemic control, and patients typically suffer a wide range of secondary health complications. Researchers and clinicians have long sought a method to replace the β cells, but these efforts have been hindered by the difficulty in developing a reliable and sustainable donor b cell source and a way to engraft them that avoids both immune-mediated allogeneic rejection and continuing autoimmune b cell destruction. In a recent report in Nature Medicine (Vegas et al. 2016), a multi-institutional team lead by Dr. Daniel G. Anderson of the Massachusetts Institute of Technology and Boston Children’s Hospital describes a new approach in which β cells differentiated in vitro from human pluripotent stem cells (hPSCs) were packaged in a modified alginate matrix and transplanted into immuno-competent C57BL/6J mice (000664) made diabetic by treatment with streptozotocin (STZ), which selectively destroys the mouse b cells. Following engraftment, the encapsulated human cells escaped xenogeneic immune destruction and maintained stable long-term glycemic control for up to six months.
Microencapsulated human β cells evade mouse immunity
Prior attempts to transplant β cells to restore glycemic control to T1D patients have used cadaveric- and porcine-derived cells. These cells typically do not maintain long-term glycemic control because they stimulate strong host-mediated immune reactions leading to cell necrosis and fibrosis. Researchers have attempted encapsulating donor β cells in a variety of materials to prevent allo- and xenogeneic rejection, but these materials themselves can stimulate innate immune responses. In their recent study, Dr. Anderson’s team used two new novel approaches. First, they developed an in vitro culture method to create human insulin-producing β cells from huPSCs (stem cell-derived β cells, SC-β). The key advantage of these cells is that large numbers can be created in culture for transplantation. Second, the researchers developed a chemically modified version of alginate microspheres that could evade innate immunity. They discovered that alginate modified with triazole-thiomorpholine dioxide (TMTD) would resist fibrosis in both mice and non-human primates. The Anderson team also examined the influence of microsphere size on glycemic control by comparing 0.5 mm and 1.5 mm unmodified alginate spheres to 1.5 mm TMTD alginate spheres. C57BL/6J (000664) mice made hyperglycemic by STZ treatment were injected intraperitoneally (IP) with 100, 250, or 1,000 SC-b encapsulated microspheres. Mice engrafted with the 0.5 mm alginate spheres showed normal blood glucose levels for about 10 days at the two highest doses tested. Mice engrafted with the 1.5 mm alginate spheres remained normoglycemic for slightly longer – for 10-15 days, and glucose was lowered in a dose dependent manner. The mice engrafted with the TMTD alginate spheres, however, maintained normal glucose levels at all three sphere doses for the full 90 days of the experiment. This experiment demonstrated that the larger TMTD-modified microspheres were most efficient at maintaining long-term normoglycemia in STZ-treated, diabetic mice.
Another important question for the Anderson team was whether the TMTD alginate microspheres would resist host innate immune responses. In a separate experiment, SC-β cell-loaded unmodified and TMTD-modified alginate microspheres were isolated from engrafted mice 14 days post-engraftment, and the presence of host immune cells adhering to the microspheres was examined by flow cytometry. Consistently fewer macrophages, neutrophils, β cells, and cytotoxic T cells were associated with TMTD alginate spheres compared to unmodified alginate spheres. The smallest (0.5 mm) unmodified spheres showed the greatest number of associated immune cells. Next, immunofluorescence was used to examine spheres for fibrosis. Unmodified microspheres showed abundant staining with a-smooth muscle actin (a-SMA), but TMTD alginate spheres did not. This was further verified by immunoblotting extracts of the recovered microspheres for a-SMA protein. Additional proteomic analyses showed that the cells obtained from TMTD alginate spheres had lower levels of multiple host-derived collagen isoforms. Together, these data demonstrate that the TMTD-modified, larger microspheres induced significantly less innate immune cell stimulation and reduced fibrosis.
Microencapsulated human b cells provide long-term glycemic control
Although their initial experiments clearly demonstrated that TMTD-modified microspheres protected the human SC-β cells from the mouse host’s immune responses and that the SC-β cells could produce enough insulin to regulate blood glucose, the Anderson team also wanted to evaluate their method’s ability to maintain normoglycemia long-term. A separate cohort of STZ-treated C57BL/6J mice were injected IP with 250 TMTD alginate microspheres and were monitored for 6 months. For the entire period, blood glucose in the treated mice was very similar to levels observed in healthy, C57BL/6J control mice. Further, when the treated mice were challenged by a glucose tolerance test at 150 days post-engraftment, they cleared the injected glucose at a rate similar to controls.
One possible explanation for the long-term glycemic control in the TMTD microsphere-treated mice was regeneration of mouse islet β cells, but quantitation of insulin from the pancreas of STZ-treated controls and STZ-treated mice implanted with unloaded TMTD alginate microspheres showed minimal insulin production. Microspheres isolated from long-term, SC-β cell-loaded TMTD microsphere-treated mice showed abundant insulin staining by immunohistochemistry (see figure), but little glucagon co-localization. The SC-β cells also retained expression of the β cell marker NKX6.1 and C-peptide, a surrogate marker for insulin secretion. These data verify that the SC-β cell cells in the injected microspheres retained their differentiated state and continued to produce insulin 6 months post-engraftment. Further, the SC-β cell-loaded TMTD alginate microspheres were able to evade mouse immunity and maintain long-term glycemic control.
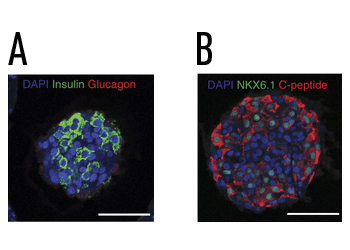
Encapsulated human SC-β cells isolated from STZ treated C57BL/6J mice 6 months post-transplant. Human insulin SC-β cells were encapsulated in a triazole-thiomorpholine dioxide (TMTD) modified alginate matrix and were transplanted IP into hyperglycemic mice. The engrafted mice maintained normoglycemia for 6 months, when the implants were removed and evaluated by immunohistochemistry. (A) SC-β cells maintained insulin expression, and were negative for glucagon. (B) The cells expressed the β cell marker NKX6.1 and were also positive for C-peptide expression, demonstrating that the SC-β cells maintained the appropriate expression and differentiation markers long-term and enabled systemic glycemic control in the recipients.
The data presented in the Vegas et al. study clearly demonstrates strong, preclinical evidence that human insulin-producing β cells can be generated in culture from pluripotent stem cells and that these cells can be used to reverse hyperglycemia. To do so, the cells must be encapsulated in a matrix that protects them from host immune cell destruction. In patients, encapsulated β cells will have to evade both allogeneic responses arising from donor/host HLA mismatches, as well as the untreated host’s continuing autoimmunity towards antigenic peptides presented on the insulin-producing SC-β cells. The Anderson team’s experiments provide hope that this approach may lead to a new treatment strategy that could lead to better glycemic control and diminished primary and secondary complications associated with type 1 diabetes.
