Cytotoxic T Cells Strong Stuff in Mothers Milk
July 6, 2016Transfer of maternal immunity to nursing young
Newborn babies are born with a naïve adaptive immune system. Although hematopoiesis occurs during development in utero, the fetus is exposed to very few antigens because the placenta and amniotic sac segregate it from the mother’s bloodstream and circulating external antigens. As a result, newborns in the postnatal period are at an immune disadvantage right at the very time when they are suddenly surrounded by new pathogenic organisms, and they remains so until their immune cell repertoire matures. Passive immunity in the form of high levels of immunoglobulins present in breast milk has been demonstrated to significantly boost newborn immunity. These soluble IgA antibodies present in breast milk can recognize epitopes previously encountered by the mother and work in concert with the infant’s regulatory T cells to stimulate B- and T-cell reactions to both clear these pathogens and stimulate memory B cell-mediated, long term immunity in the infant. More recent studies have demonstrated that breast milk carries additional immune constituents, including macrophages, neutrophils, and lymphocytes. How these maternal leukocytes function and contribute to immune activity and immune cell development in the newborn is not fully understood. In a recentPLoSONE study, a collaborative team of researchers led by Dr. Amale Laouar from Rutgers University in New Jersey and Dr. Yasmina Laouar from the University of Michigan School of Medicine, investigated the trafficking of maternal leukocytes to organs of suckling young using mouse fostering models. Their data demonstrates that maternal cytotoxic CD8+ T cells and other immune cell types home to Peyer’s patches in the fetal intestine where they can participate in antigen targeting of gut-borne pathogens. These observations broaden our understanding of newborn immunity and reinforce the benefits of breast feeding in preventing childhood diseases.
Mouse fostering experiments demonstrate leukocyte trafficking to Peyer’s patches
In order to examine where maternally-derived leukocytes reside following transmission to suckling young, Laouar teams performed a number of fostering experiments (Fig 1). First, they fostered wild-type C57BL/6J (000664) pups using mothers that foster mothers express a GFP transgene regulated by the human ubiquitin C gene promoter (C57BL/6-Tg(UBC-GFP)30Scha/J,004353). In these C57BL/6-GFPtg mice, nearly all cells in all tissues express GFP, allowing maternal cells to be visualized easily in non-GFP-expressing wild-type C57BL/6J foster pups. At weaning (21 days), maternal GFP-positive (GFP+) cells were preferentially located in intestinal Peyer’s patches in the foster pups. These small tissues are sites of immune cell development, and have been associated with gut immunity. Very little GFP fluorescence was observed in other tissues in the fostered pups, and once breastfeeding from the GFP+ dams ended, GFP-positivity in the Peyer’s patches rapidly diminished, and was undetectable in adult mice.
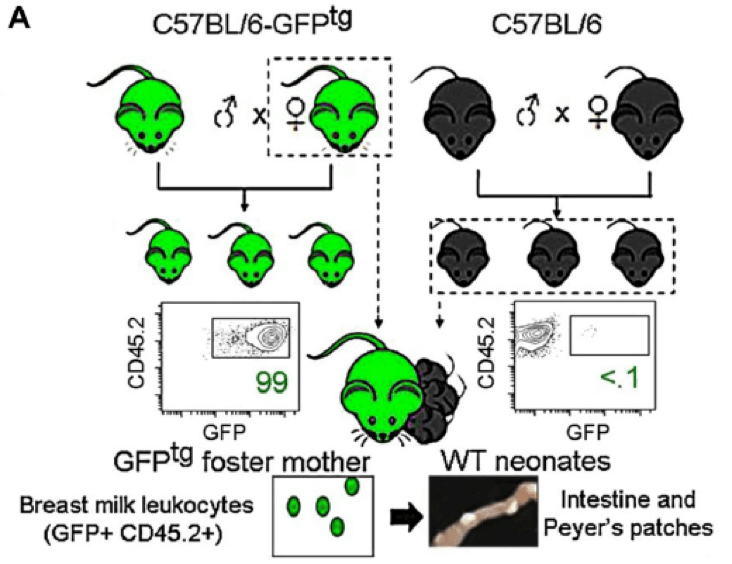
Figure 1. Schematic for fostering experiments. C57BL/6-GFPtg (004353) mice express a GFP transgene in all cells, so when wild-type, non-transgenic C57BL/6J pups were fostered to the C57BL/6-GFPtg dams, maternally-derived cells could be visualized in pup tissue by direct, fluorescence microscopy. Intestinal Peyer’s patches in fostered pups showed bright GFP fluorescence at weaning, indicating that maternal cells were present. Adapted from Cabinian et al, 2016.
This particular fostering scheme, however, although useful for visualizing maternal cells, is not representative of the situation in humans in which mother and child are not HLA-matched due to HLA contributions from both mother and father in the infant. To better model the human scenario, the Laouar team also fostered BALB/cJ (000651) pups, which express the MHC haplotype, H2d, to the MHC-H2b-expressing C57BL/6-GFPtg dams. In this fostering model, GFP-expressing maternal cells were again detected at weaning in Peyer’s patches in fostered pups, and their longevity was similar to that observed in the MHC-matched experiment: maternally-derived GFP+ cells were rapidly lost after weaning and were no longer detectable in pups or adult mice after weaning. These data demonstrate that maternal cells are successfully transmitted to suckling young and that they localize to known immune organs (Peyer’s patches), even when the mother and offspring are mismatched at their MHC loci.
Transferred maternal leukocytes are predominantly active cytotoxic T cells
To determine which immune cells were transferred from dams to their foster offspring, the investigators isolated cells from foster pups’ Peyer’s patches, and sorted the present immune cells by flow cytometry. These experiments revealed that although the majority of cells present (80%, roughly) in the milk were myeloid cells (Gr-1+, CD11b+, and CD11c+), the majority (60%) of cells present in Peyer’s patches were CD3+ T lymphocytes - predominantly CD8+ cytotoxic T cells (CTLs). Further, comparison of surface markers on these CTLs compared to those on circulating CTLs from the foster dams revealed that the Peyer’s patch-associated CTLs transferred from breast milk expressed high levels of integrin α4β7 and chemokine receptor CCR9, which are characteristic gut-homing signals. These data suggest that maternal CTLs in breast milk are programmed to home to intestinal Peyer’s patches. The transferred CTLs, also, were approximately 4-fold more potent than infant-derived CTLs in T lymphocyte activation assays following stimulation with PMA/Ionomycin or anti-CD3/anti-CD28 antibodies. These results demonstrate that mothers transfer tissue-targeted, active CTLs to nursing young, which are primed and ready to combat encountered pathogens immediately after birth, bridging the vulnerable time in which the infant’s immune system is still maturing. This study provides new context to our current understanding of passive immunity transferred from mother to young through breast milk, and may contribute to new ways of preventing childhood diseases and improving post-natal care.
Reference
Cabinian A et al. 2016. Transfer of maternal immune cells by breastfeeding: maternal cytotoxic T lymphocytes present in breast milk localize in the Peyer’s patches of the nursed infant. PLoSONE 11(6):e0156762. PMID:27285085.
