Young Blood May Improve Your Memory
eNews | August 5, 2015
β2-microglobulin in aging blood impairs learning and memory
Recent findings in aging research demonstrate there are factors in the blood of young versus old mice that influence multiple aging phenotypes. When the circulatory systems of young and old mice are connected through a procedure called parabiosis cognitive function and neurogenesis declines in young mice and improves in old mice. New research reported inNature Medicine (Smith et al., 2015) demonstrates that β2-microglobulin (β2M), a subunit of the major histocompatibility complex class I (MHC I) molecule, increases in the blood during aging and causes diminished cognitive function and neurogenesis. The aging-related defects caused by β2M appear reversible implicating this molecule as a potential therapeutic target to mitigate a wide range of neurodegenerative diseases.
β2M accumulates in blood as we age
MHC I is expressed on the surface of nearly all cells, and plays a key role in immune function. Interestingly, MHC I is also involved in brain development, neuronal circuitry plasticity, and behavior. Age-related increases in β2M are found in human blood as well as in cerebrospinal fluid and elevated β2M is associated with human neurodegenerative disease. When the circulatory systems of young (3 months) and old (18 months) C57BL/6J (000664) mice were connected through heterochronic parabiosis (see figure), β2M from the old mouse was transferred to the younger mouse, and accumulated in the younger animal's brain.
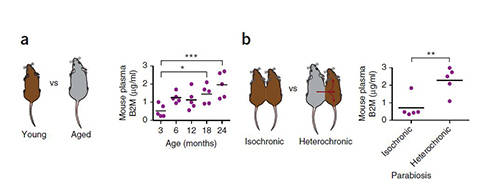
Figure 1. β2M increases in the blood as mice age and causes cognitive decline and diminished neurogenesis in young mice following parabiosis. β2M progressively increases in the plasma of mice as they age (a). β2M from old mice increases in young animals following heterochronic parabiosis (b). Mice treated with β2M injections show impaired learning and memory and a decline in new neurons in the dentate gyrus (not shown). Adapted from Smith, J., et al. 2015. (Nature Medicine. Jul 6. doi: 10.1038/nm.3898)
β2M increases in the blood as mice age and causes cognitive decline and diminished neurogenesis in young mice following parabiosis. β2M progressively increases in the plasma of mice as they age (a). β2M from old mice increases in young animals following heterochronic parabiosis (b). Mice treated with β2M injections show impaired learning and memory and a decline in new neurons in the dentate gyrus (not shown). Adapted from Smith, J., et al. 2015. (
Jul 6. doi: 10.1038/nm.3898)
Injection of β2M impairs learning and memory
When young mice were injected intravenously with β2M 5 times over 12 days, the animals showed a decline in learning and memory in the radial arm water maze (RAWM) assay and a reduced incidence of freezing in the contextual fear conditioning assay. The β2M-injected mice also showed a decrease in hippocampal neurogenesis, based on a reduced number of doublecortin positive neurons, nestin-positive and minichromosome maintenance type 2-positive progenitors, and lower BrdU incorporation in cells of the dentate gyrus.
Smith et al. next tested whether local exposure to β2M has the same effect as systemic exposure. Using stereotaxic injection, β2M was delivered directly into both lateral ventricles of the brain in young mice and cognitive function was measured 6 days later. Compared to vehicle treated controls, the β2M-treated mice showed more errors in their RAWN performance and decreased freezing time in contextual fear conditioning. When behavioral testing was done 30 days post-treatment, β2M-treated mice did not show significant differences from controls, suggesting that the impact of β2M exposure may be reversible.
The role of MHC I cell surface expression
A remaining question was whether cell surface expression of MHC I was necessary for the development of aging phenotypes induced by soluble β2M. Mice knocked out for the geneTap1 (B6.129S2-Tap1tm1ArpM/J, 002944) are defective in the stable assembly and intracellular transport of class I molecules, and show severely reduced levels of class I on their cell surfaces. When β2M was administered to youngTap1 -/- mice via bilateral ventricle injection, no changes in the animal's cognitive functions were observed when compared to vehicle-treated controls. The authors also examined the role of β2M surface expression in neurogenesis. Young wild-type C57BL/6J and Tap1 -/- mice were injected with vehicle in the dentate gyrus (DG) of one hemisphere and with β2M in the contralateral dentate gyrus. Hippocampal sections were examined, and only the β2M-treated DGs from wild-type mice showed evidence for decreased neurogenesis. In contrast, β2M-injected DGs from similarly treated Tap1 -/- mice showed no decline in neurogenesis compared to the vehicle-treated contralateral DGs. Finally, brains from young Tap1 -/- that were exposed to increase circulatory β2M via heterochronic parabiosis with old wild-type mice demonstrated no decline in neurogenesis. These results demonstrate that cell surface expression of MHC I is required for the age-related cognitive decline induced by soluble β2M.
Aged β2M -/- mice have improved cognitive function
Smith et al. also examined whether the absence of cell surface β2M alters the progression of cognitive decline in aged mice and found that young wild-type C57BL/6J andB2M -/- (B6.129P2-B2mtm1Unc/J, 002087) mice performed similarly in behavioral assays. Further, the B2M -/- mice showed no evidence of diminished neurodegeneration. Aged B2M -/- mice, however, showed significantly enhanced spatial learning and memory compared to aged wild-type controls. The aged B2M -/- mice also showed no decrease in neurogenesis. Therefore, the absence of β2M expression prevents age-related loss of cognitive function and neurogenesis.
In conclusion, the findings of Smith et al. clearly implicate a role for β2M in age-related cognitive decline and in the associated reduced neuronal proliferation and survival. Young mice exposed to β2M show a cognitive decline that improves following a recovery period, providing hope that therapies designed to diminish soluble β2M may be able to improve cognitive function in the elderly. Additional experiments will be necessary to determine if β2M is a viable therapeutic target for the more severe age-related cognitive disorders and neurodegenerative diseases.
