Summary: This resource answers frequently asked questions about the different biomarkers included in HRD testing.
By Clinical Education at JAX | June 2024
Summary: This resource answers frequently asked questions about the different biomarkers included in HRD testing.
By Clinical Education at JAX | June 2024
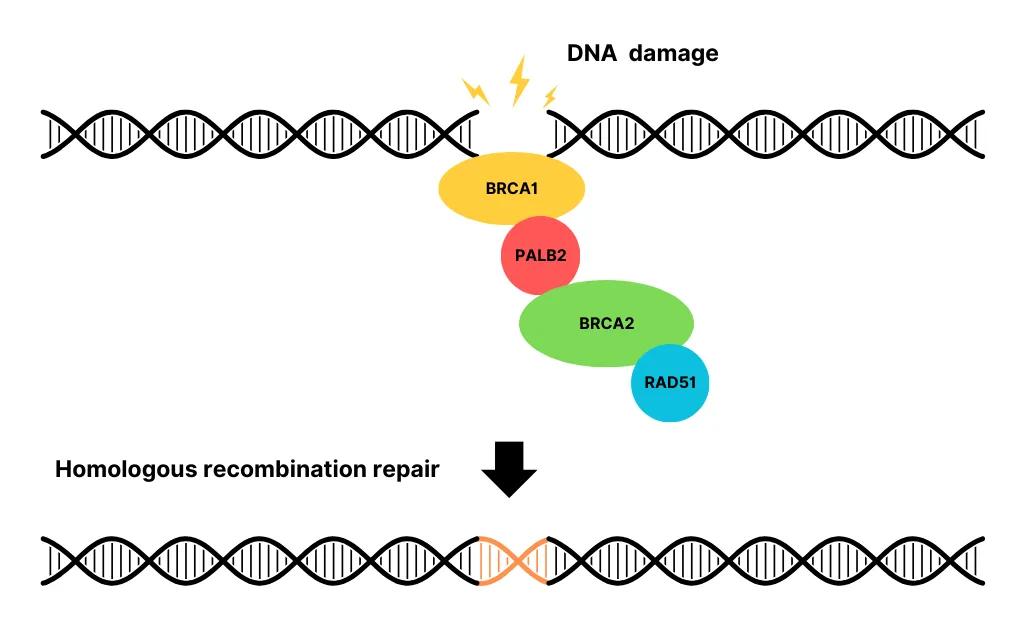
Homologous recombination deficiency is the result of a dysfunctional DNA repair process known as homologous recombination repair (HRR). HRR is responsible for repairing DNA double-strand breaks. The HRR protein complex includes BRCA1, BRCA2, RAD51, PALB2, BARD1, and others (Figure 1). When genes involved in HRR, mainly BRCA1/2, develop mutations, the DNA repair process is impaired, leading to wide-spread chromosomal structural changes across the cell. This accumulation of chromosomal variants is also known as genomic instability.
Figure 1: Homologous recombination repair.
Multiple biomarkers can be used to assess HRD, but there is no consensus about which biomarkers are the best indicators of HRD. Labs determine which biomarkers to include on the tests independently. In general, HRD tests assess the cause of HRD (germline or somatic pathogenic variants in BRCA1/2, or other genes involved in HRR) or the effect of HRD (genomic instability) (Figure 2).
Figure 2: Biomarkers included in HRD testing
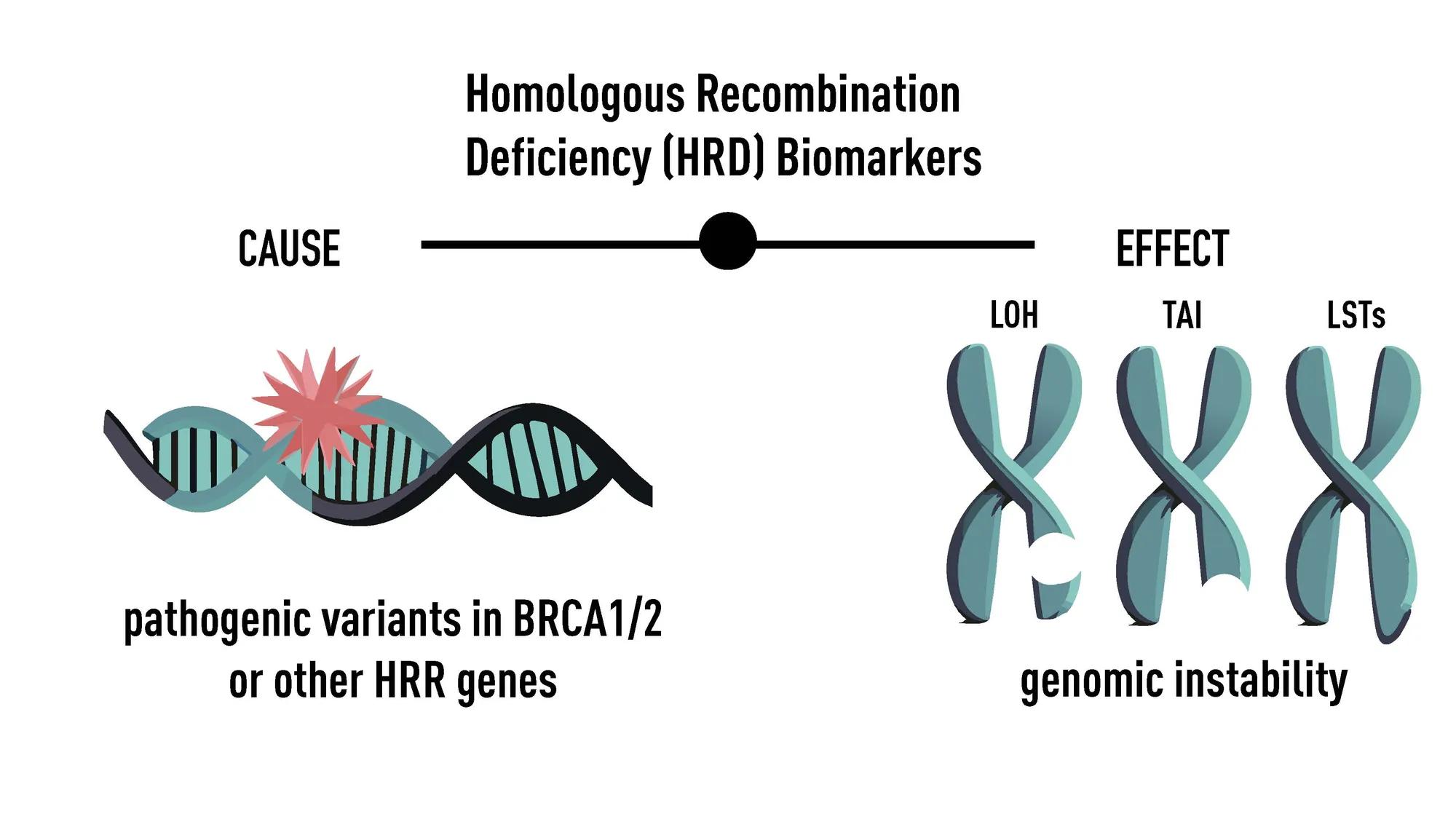
HRD is the presence of a dysfunctional homologous recombination repair (HRR) pathway. An HRD score is a proprietary lab-specific algorithm based on one or multiple HRD biomarkers.
Loss of Heterozygosity (LOH) is one of many biomarkers that assess the effect of HRD. LOH is a measure of genomic instability that results from the loss of one copy of a gene or chromosomal region. HRD score can include LOH, but this will vary from lab to lab.
Some testing labs only assess targetable BRCA1/2 variants. Other labs will report a combination of HRD biomarkers, which can include gene variants as well as genomic instability scores, such as LOH or HRD.
For certain cancer types (e.g., ovarian), identifying a cancer with HRD can help guide treatment decisions, especially in the selection of therapies such as PARP inhibitors (PARPi). PARPi are FDA-approved in some cancers with HRD (ovarian, breast, pancreatic, prostate). Use of PARPi in the setting of HRD further inhibits DNA repair, leading to cell death of the cancer cells and, potentially, improved patient outcomes.
See PARPi Inhibitors: Overview and Indications for more information about the benefit of PARPi in different scenarios.
HRD results are reported differently among laboratories, depending on the biomarkers used in testing. HRD may be reported as positive or negative, or individual HRD biomarkers may be reported separately, such as the presence of BRCA1/2 variants or LOH score.
Details of test parameters are often available within the test report, on the laboratory website, or upon request from the lab representative. Genomic tumor boards and consultation with genetics professionals can be useful sources of peer consultation. Referral to appropriate specialists, like a genetic counselor, may be considered with suspected germline findings.
PARP Inhibitors: Overview and Indications. Discusses FDA-approved indications for PARPi for maintenance therapy and cancer treatment.
Exploring Cancer Biomarker Testing (CME|CNE). Learn about benefits, limitations, and challenges of using cancer biomarker testing.
Interpreting Cancer Biomarker Testing – When is Additional Testing Needed? (CME|CNE). Learn when additional cancer biomarker testing is indicated for further evaluation of genome-informed therapy.
Indications for Germline Testing after Genomic Tumor Testing (CME|CNE). Identifies genetic red flags to inform personal and family history risk assessment and genomic tumor test results that are suggestive of a germline variant.
Mangogna A, Munari G, Pepe F, et al. Homologous Recombination Deficiency in Ovarian Cancer: from the Biological Rationale to Current Diagnostic Approaches. J Pers Med. 2023 Feb 2;13(2):284.
All information in this resource is provided for educational purposes only.