Achieving Relevant Preclinical Characterization of CAR T Cell Therapies
Blog Post | April 8, 2022

Introduction
Since the first chimeric antigen receptor (CAR) T cell therapy received FDA approval in 2017, this promising immunotherapy modality has only continued to gather momentum. Existing CAR T cell therapies have shown great success in treating blood cancers, and researchers are exploring ways to expand applications into solid tumors and beyond. CAR T cell therapies can offer hope to patients for whom other treatments have failed.
However, since CAR T cells are living immune cells that are significantly modified before being reintroduced into the patient, their interactions within the body can be difficult to control and predict. T cells are killing machines that can damage healthy tissue if there are on-target but off-tumor interactions (normal cells expressing the tumor-associated antigen). Furthermore, these immune cells can release or cause other cells to release excessive amounts of signaling proteins known as cytokines, potentially triggering a cascading effect called cytokine release syndrome (CRS). CRS can be difficult to control and has systemic symptoms that range from fever and inflammation to organ failure and death. 77-93 percent of leukemia patients and 37-93 percent of lymphoma patients experience CRS after receiving CAR T cell treatment, and approximately 50 percent of patients involved in early CAR T cell therapy clinical trials required intensive care management.1 On top of the risk to patients, trial failures and delays resulting from these events can cause significant loss of time, financial resources, and public goodwill for biopharmaceutical developers.
To avoid these risks and setbacks, scientists need a preclinical research platform that will deliver fast, reproducible, relevant, and actionable results without endangering human patients. By studying the behavior and effects of CAR T cells in vivo using humanized mouse models, researchers can gain a comprehensive understanding of how these therapies interact with complete biological systems in a dose-dependent and cell donor-dependent manner. Armed with this information, clinicians and therapeutic developers can have greater confidence that a CAR T cell therapy will be both safe and effective for each patient who receives it, including clinical trial participants.
Overcoming the Limitations of Other Preclinical CAR T Studies
Existing research methods for studying CAR T cells outside of human trial subjects fail to capture the population diversity, systemic interactions, and unique human cell characteristics that affect the toxicity and efficacy of CAR T cell therapies observed in the clinic. Here, we examine the constraints and challenges inherent in each study type and how to overcome them with in vivo studies using immune humanized mouse models.
In Vitro Assays Can Only Go So Far
In vitro CAR T cell characterization assays typically involve culturing CAR T cells with tumor cells and human peripheral blood mononuclear cells (PBMCs). This process allows scientists to measure the CAR T cells’ effectiveness at killing individual tumor cells, as well as safety-related outcomes such as cytotoxicity and cytokine production.
These types of studies are relatively efficient and inexpensive, and are therefore useful for providing rapid feedback at early stages of CAR T cell development. However, toxicities like CRS are cascading responses that impact systems outside the circulating blood cells that initially interact with the administered CAR T cells after they are injected. Because of this systemic nature, scientists cannot use in vitro studies to detect off-target effects or model downstream effects such as organ damage and neurotoxicities within a closed system. In vitro studies cannot deliver a comprehensive picture of how CAR T cell treatment might affect living patients.
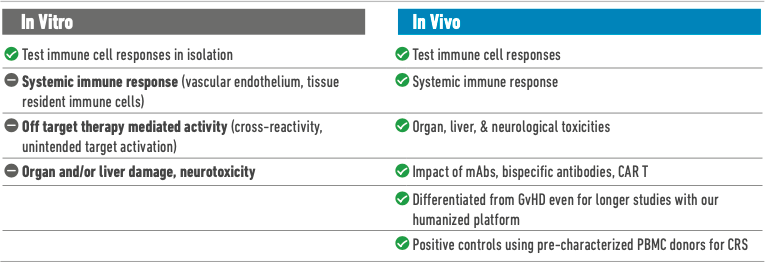
Limitations of Current In Vivo Models
Unlike in vitro studies, in vivo models can approximate the downstream outcomes of systemic adverse responses, allowing researchers to study organ effects and measure health indicators such as overall change in body weight. In the past, however, preclinical studies in mice, non-human primates, and other animals have failed to predict dangerous CRS responses that were later observed in human trial subjects, because a severe immune response can sometimes be tied to the expression of a receptor that is unique to human immune cells.2
Immunodeficient mice, when used for CAR T cell therapy studies without modification, fail to model systemic immune response. This is because they lack key components of an innate immune system—murine or human—and will therefore not exhibit representative cytokine release levels or other clinically relevant phenotypes.
CD34+ mice are a popular option for preclinical immunotherapy evaluation because they develop human immune cells representative of both lymphoid and myeloid lineages and exhibit useful primary immune responses. However, in these models, T cells are educated on the mouse murine major histocompatibility complex (MHC) rather than on the human leukocyte antigen (HLA) normally expressed on human thymic epithelial cells. The human T cell response in these mice therefore lacks some clinical relevance, as do many subsequent downstream effects.3
Historically, non-human primate trials were considered the gold standard of preclinical drug safety and efficacy testing because these animals most closely modeled human physiology and disease progression. However, non-human primate trials are extremely expensive and time-intensive and involve serious ethical concerns. The replacement of NHPs with in vitro assays risks missing cell non-autonomous toxic interactions that might happen only in vivo, such as the mechanism underlying CRS. On the other hand, most in vivo preclinical models (such as rodents, dogs, and rabbits) lack the human-specific drug target and have very little translational value. Furthermore, now that scientists can obtain reproducible results in humanized mice, non-human primates are no longer the closest preclinical approximation of a functional human immune system.
The Answer: PBMC-Humanized Mouse Models
To improve preclinical research and surmount the issues discussed above, scientists at The Jackson Laboratory (JAX) developed a 16-day in vivo assay for the analysis of therapy-induced CRS and other responses in mice humanized with pre-characterized adult PBMCs. This humanized mouse platform is an ideal tool for understanding how a functional human immune system will respond to CAR T cell administration. By using immunodeficient NSG mice deficient in murine MHC class I and II (NSG-MHC I/II KO) as the genetic background for PBMC humanization, it is possible to minimize the risk of xenogeneic graft vs. host disease (GvHD), so scientists can have greater confidence that any observed toxicities result from CAR T cell therapy rather than other confounding factors.4 This tool offers scientists the chance to observe HLA-trained human immune cells reacting to CAR T cells, all while circulating and interacting with cells and tissues from all normal organs and biological systems.
After PBMC injection and subsequent administration of CAR T cells or control treatments, researchers can monitor the health of each mouse by serially measuring body weight and temperature and quantifying human cytokines at key time points. At the end of the experiment, scientists can also obtain tissue samples to study organ infiltration, liver toxicity, cardiotoxicity, neurotoxicity, and other downstream effects. The PBMC-humanized mice also accept tumor engraftment, making it possible to simultaneously study toxicity and efficacy by measuring tumor growth inhibition and other markers. In fact, the presence of the tumor and its target molecule is often necessary to initiate cytokine mediated systemic toxicity.
In addition to being more relevant to human biology than other in vivo assays, studies that use PBMC-humanized mice deliver higher-quality data than in vitro assays. In head-to-head comparisons, JAX studies using these mice yielded more reproducible and potentially clinically relevant results.5 In the following sections, we will explore the research possibilities unlocked by this powerful tool.
Optimal Preclinical Data: Predicting Efficacy and Comprehensive Toxicity in a Single Model
Answering multiple research questions with studies in a single animal model can increase the efficiency of a therapeutic development pipeline. Validation studies have demonstrated that PBMC-humanized mice can rapidly deliver high-quality data about dosing, characterize the biological mechanisms underlying various toxicities, and offer insight into factors that contribute to differing clinical responses in diverse populations.
Modeling Dose-Dependent Efficacy
Since humanized mouse models readily accept tumor engraftment, researchers can compare the efficacy of different CAR T cell therapies and different doses of the same therapy. Efficacy can be quantified using multiple metrics, including direct measurement of tumor growth and observation of health effects such as weight loss.
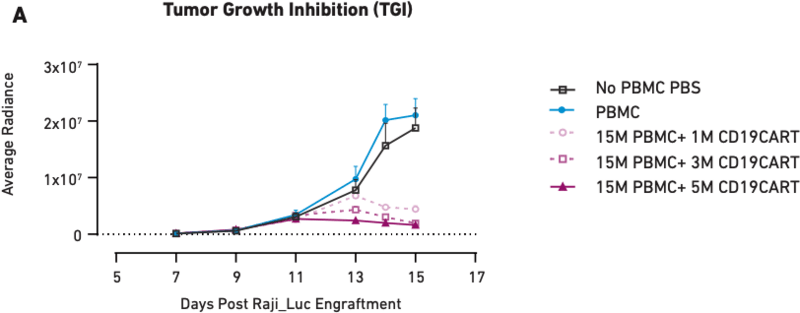
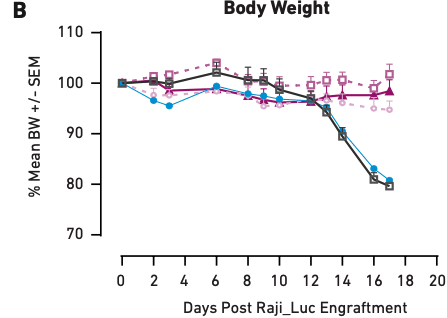
Figure 1 These graphs show tumor growth inhibition (A) and body weight measurements (B) in mice humanized with PBMCs from a single donor and treated with different amounts of CAR T cells. PBS and PBMCs alone were the negative controls. Here, loss of body weight is associated with increased tumor burden.
Figure 1A demonstrates that as CAR T cell dose increases from one million cells to five million cells, tumor growth inhibition also increases, showing a correlation between therapeutic dosage and reduction in tumor growth. Similar correlations have been observed in clinical applications of CAR T cell therapies; therefore, the mice accurately modeled direct outcomes. The same is true of health effects—Figure 1B shows that the mice that did not receive CAR T cell treatment (PBS and PBMC only) experienced significant tumor burden-induced weight loss, while the three CAR T cell therapy arms of the study showed little or no loss of body weight, consistent with the aforementioned impact on tumor growth. Together, these data demonstrate that PBMC-humanized mice exhibit dose-dependent responses to CAR T cell therapies that are in line with clinical expectations.
Observing Robust Toxicities
In supplementary validation studies of the PBMC-humanized mouse platform, researchers found that they could generate stronger toxic effects by increasing the number of PBMCs used to humanize the mice. Doing so increased the quantity of circulating CD19+ B cells, which were the targets of the autologous CAR T cells administered in the study.
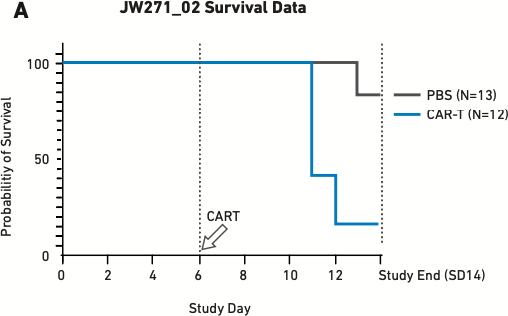
Figure 2 These figures show survival (A) and cytokine release data (B) for mice humanized with 17x106 PBMCs six days before being treated with 5x106 autologous CAR T cells—the lowest CAR T cell dose used in other validation studies. PBS is the negative control.
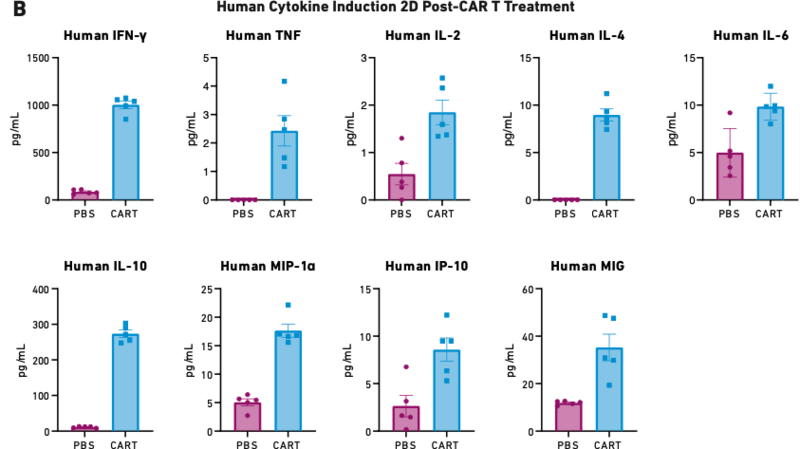
As Figure 2A demonstrates, 15 percent of mice treated with CAR T cells reached endpoint prior to the study’s completion on day 14. The mice treated with CAR T cells also exhibited extremely high levels of cytokine release (Figure 2B), which triggered severe CRS and other downstream toxicities.
The ability to reliably produce a robust toxicity response in PBMC-humanized mice offers researchers a reliable tool for studying CRS and other CAR T cell-related toxicities at multiple biological levels, from whole organs down to molecular mechanisms. Such studies could shed light on possible prevention or treatment strategies for when these toxicities occur in clinical settings.
Determining Donor-Specific Safety Profiles
Researchers have observed reproducible variation in response to immune stimulus in mice humanized with PBMCs from different donors.5 This donor-specific variation can be used to model diverse individual responses to CAR T cell therapy. After observing different effects, scientists can compare the biological profiles of pre-characterized human donors to identify factors that potentially underlie an elevated risk of toxicity. This type of study could inform CAR T cell therapy trial inclusion/exclusion criteria, and ultimately provide guidance for clinicians making an initial judgment on whether CAR T cell therapy is the best treatment option for their patients.
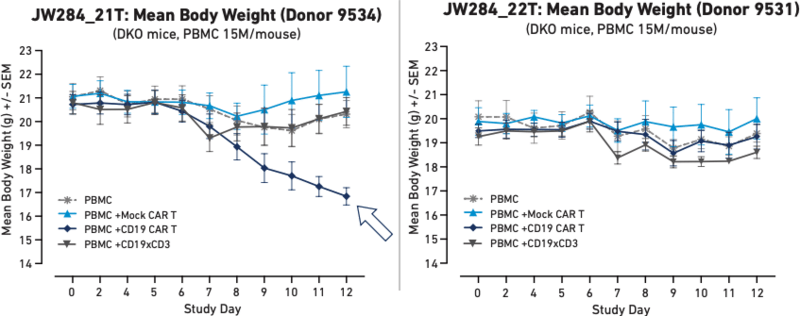
Figure 3 These graphs represent body weight in humanized mice with different PBMC donors following the administration of Mock CAR T, CD19 CAR T, or a CD19xCD3 bispecific T cell engager. Here, donor 9534 developed a systemic toxic cytokine response when treated with CAR T that led to severe weight loss.
Figure 3 demonstrates different health outcomes observed in mice humanized with two different PBMC donors. Mice humanized with PBMCs from Donor 9534 (Figure 3A) experienced a sharp decrease in body weight following autologous CAR T cell treatment, whereas mice humanized with PBMCs from Donor 9531 (Figure 3B) did not show the same decrease in body weight. These results demonstrate that PBMC-humanized mice are capable of modeling systemic effects that differ between human donors.
This reflection of diversity allows researchers to obtain more holistic data ahead of clinical implementation of CAR T cell therapies. This in vivo platform could possibly support a precision medicine approach to treatment selection for cancer and other diseases that may someday be addressed using CAR T cells. A physician could use PBMCs from a patient to humanize mice for study prior to administering clinical treatment to obtain a personalized prediction of whether CAR T cell therapy will be both safe and effective for that particular patient. If a humanized mouse study reveals that the patient is not a good candidate for CAR T cell therapy, the physician can spare the patient unnecessary risk and pivot quickly to other treatment options to avoid wasting precious time.
Expanding the Horizons of CAR T Cell Therapies
PBMC-humanized mice offer an ideal platform for developing, testing, and refining new types of CAR T cell therapies without endangering human clinical trial participants.
Allogeneic CAR T Cells
All the CAR T cell therapies in use in clinics today are autologous, meaning that they are custom-made for each patient by removing T cells from their body, genetically modifying them to express the CAR protein, expanding the cells, and returning them to the patient. However, this individualized protocol means that the manufacturing process is slow and extremely costly, decreasing accessibility and delaying potentially life-saving treatment.
Many biopharmaceutical researchers are actively attempting to develop allogeneic CAR T cells, which would offer an off-the-shelf treatment option that could be delivered faster and less costly. However, these cells are foreign to patients’ bodies and therefore are at risk of being destroyed without therapeutic benefit or causing dangerous immune responses such as severe CRS or GvHD. As a result, these cells have proven difficult to test without posing unacceptable risk to clinical trial patients.
PBMC-humanized mice allow scientists to test allogeneic CAR T cells in a diverse population to obtain a more comprehensive and accurate profile of how different patient groups may respond in the clinic. This platform could also be used to generate data that would inform the design of allogeneic CAR T cells to minimize reactivity with the widest possible range of human immune systems.
Modified CAR Structures
Studies have indicated that the structure and distribution of CARs on CAR T cells can impact their behavior, and potentially their efficacy.6 High expression of CAR proteins can result in antigen-independent tonic signaling and activation, while the structure of individual domains can affect factors such as target recognition and persistence. Researchers could modify these structural characteristics using gene engineering and create novel forms of CAR T cells that could overcome current challenges such as infiltrating solid tumor microenvironments.
PBMC-humanized mice provide a preclinical platform for researchers to experiment with how such structural changes impact the systemic in vivo behavior of the cells—efficacy, interaction with other immune cells, and more.
Conclusion
PBMC-humanized mice are ideal for CAR T cell therapy characterization, safety, and efficacy testing. This platform enables researchers to model human immune responses to CAR T cell treatment in a comprehensive manner that overcomes the limitations of other assays, such as cost, speed, ethical concerns, and failure to capture human-specific systemic responses. These mice accept engraftment of tumors to allow efficacy testing alongside toxicity analysis, and their responses to CAR T cell administration in validation studies suggest that they can deliver translationally-relevant results for a wide range of research applications, including the development of new types of CAR T cells.
By working with an experienced research partner that delivers end-to-end support from study design to data analysis, biopharmaceutical leaders can use these mouse models to usher in a new area of immunotherapies more rapidly while keeping patients and clinical trial participants safe.
References
1 Santomasso, B., Bachier, C., Westin, J., Rezvani, K., & Shpall, E. J. (2019, May 17). The other side of CAR T-cell therapy: Cytokine release syndrome, neurologic toxicity, and financial burden. American Society of Clinical Oncology Educational Book, (39), 433–444. https://ascopubs.org/doi/full/10.1200/EDBK_238691
2 Eastwood, D., Findlay, L., Poole, S., Bird, C., Wadhwa, M., Moore, M., Burns, C., Thorpe, R., & Stebbings, R. (2010, October). Monoclonal antibody TGN1412 trial failure explained by species differences in CD28 expression on CD4+ effector memory T-cells. British Journal of Pharmacology, 161(3), 512–526. https://www.ncbi.nlm.nih.gov/pmc/ articles/PMC2990151
3 Matas-Céspedes A, Brown L, Mahbubani KT, Bareham B, Higgins J, Curran M, de Haan L, Lapointe JM, Stebbings R, Saeb-Parsy K. Use of human splenocytes in an innovative humanised mouse model for prediction of immunotherapy-induced cytokine release syndrome. Clin Transl Immunology. 2020 Nov 4;9(11):e1202. doi: 10.1002/cti2.1202. PMID: 33173582; PMCID: PMC7641894.
4 Brehm, M. A., Kenney, L. L., Wiles, M. V., Low, B. E., Tisch, R. M., Burzenski, L., Mueller, C., Greiner, D. L., & Shultz, L. D. (2019). Lack of acute xenogeneic graft- versus-host disease, but retention of T-cell function following engraftment of human peripheral blood mononuclear cells in NSG mice deficient in MHC class I and II expression. FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 33(3), 3137–3151. https://doi.org/10.1096/fj.201800636R
5 Ye, C., Yang, H., Cheng, M., Shultz, L. D., Greiner, D. L., Brehm, M. A., & Keck, J. G. (2020, August 9). A rapid, sensitive, and reproducible in vivo PBMC humanized murine model for determining therapeutic-related cytokine release syndrome. The FASEB Journal, 34(9), 12963–12975. https://faseb.onlinelibrary.wiley.com/doi/ 10.1096/fj.202001203R
6 Stoiber, S., Cadilha, B. L., Benmebarek, M. R., Lesch, S., Endres, S., & Kobold, S. (2019). Limitations in the Design of Chimeric Antigen Receptors for Cancer Therapy. Cells, 8(5), 472. https://doi.org/10.3390/cells8050472
