Working with diabetic mice: 10 Do’s and Don’ts you shouldn’t ignore

Working with diabetic mice presents several challenges. Here we point out the 10 most recommended Do’s and Don’ts so you can streamline your experiments, reduce variability and get consistent results when working with the most popular mouse models of diabetes.
DO
1. Check levels of water often to ensure sufficient supply.
Diabetic mice drink more water than wt mice.
2. Make sure the mice can reach water and food.
Obese mice have a harder time to access the feeders and water supply compared to controls.
3. Provide extra food.
If you are working with hyperphagic mice such as ob/ob and db/db strains.
4. Provide extra bedding.
Mouse models of diabetes typically urinate more than wt mice.
5. Change bedding more often (~ twice a week).
Consider using cellulose based bedding such as ALPHA-Dri® for superior absorbency and ammonia control when working with polyuric strains.
6. Handle the mice with extra care.
Stressing them out may delay the onset of the disease and make the phenotype less severe.
7. Be consistent with the age of the mice used for you studies.
For several mouse models of diabetes, the course of the disease is age-dependent.
8. Be consistent with the gender used for your studies.
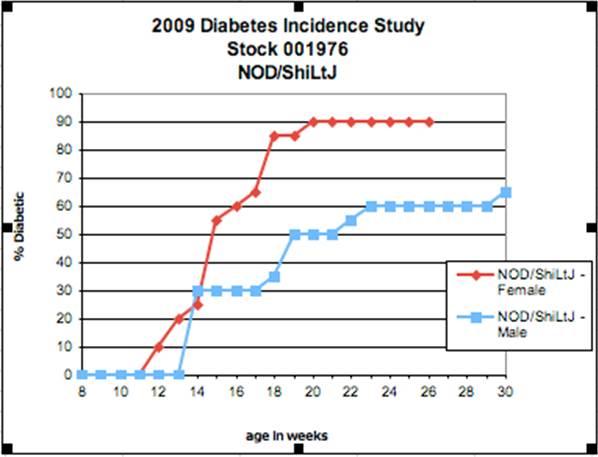
Often the penetrance and the disease severity differ between males and females. For example at 20 weeks of age, only ~50% of NOD/ShiLtJ (stock #001976) males will actually become diabetic, compared to 90% of age-matched females from the same strain.
9. Do a power analysis to determine sample size.
Include extra mice to account for non-responders and outliers. Make sure that you have enough mice (n) that express a specific phenotype to ensure statistical significance of your experiments.
10. Consider a rolling enrollment experimental design if you don’t have a large colony.
Asynchronous studies are often required for biological model of diabetes, but induced models (such as STZ ablation of beta cells) can often be run synchronously.
DON'T
1. Avoid stressing out the mice
Diabetic phenotypes in mice are highly sensitive to distress. Stress levels may interfere with gaining body weight and developing hyperglycemia (this is extremely important for lep ob/ob and lepr db/db mice models of Type 2 diabetes). Taking extra precautions will maximize phenotypic stability and minimize mouse to mouse variability.
2. Do not underestimate the benefits of adding enrichment to the cages.
Plastic Mouse Houses or Igloos, soft fibrous nesting material, chewing materials such as Nylabones®, minimize distress and promote normal behaviors.
3. Avoid neglecting the health status of the mouse room.
The presence of pathogens can delay or prevent the development of diabetes (this is extremely important when working with NOD mouse colonies).
4. Do not measure glucose levels at random times.
The best time to measure high glucose levels is early in the morning (after a good night of feeding and fun).
5. Don’t collect blood from mice using different routes.
Glucose levels vary depending on the blood collection site (retro-orbital vs tail vein).
6. Not all the mice will be hyperglycemic at one point in time (even if they are age matched).
Start out with extra mice so you can compensate for this.
7. Avoid using homozygous (lep ob/ob or lepr db/db) mice as breeders.
Fat diabetic mice have other activities in mind (eating!). Plus, homozygous mice are infertile.
8. Do not add their food in their containers and forget about it.
As Type 2 db mice become obese, it is helpful to place additional food on the floor of the cage to ensure that the mice will have easy access to it, even when they become really heavy.
9. If you are buying mice for your studies, avoid starting your experiments as soon as your mice get to your lab.
The acclimation period is critical. How long the acclimation period needs to be can vary, but it may take as long as 2 weeks. During this acclimation period, the mice are able to recover from travel, adjust to the new environment.
10. Don’t mix and match mice with the same mutation in different backgrounds.
For example mice with the Lepr mutation in the C57BLKS genetic background (stock #000642) DO progress to the late stages of diabetes with the development of islet atrophy. They are severely hyperglycemic and become progressively worse over time. On the other hand, mice with the same mutation on the C57BL/6J genetic background (stock #000697) DO NOT progress to the late stages of diabetes such as the development of islet atrophy. These mice are useful as a transient model of early stages of diabetes and their phenotype normalizes by 14-16 weeks of age.
Do you have other tips that you want to share with the Research Community? Please post your comments below.
