The Food and Drug Administration has approved several new drugs in the past decade for acute leukemia. But despite these new treatments, disease relapse remains a challenge as many of these therapeutic approaches inadequately prevent or overcome drug resistance.
While only a few DNA mutations are major contributors to relapse, The Jackson Laboratory (JAX) Assistant Professor Eric Wang, Ph.D., argues in Trends in Cancer that nongenetic mechanisms of cancer development may represent therapeutic vulnerabilities that can be exploited to overcome drug resistance in acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL).
“To date, genetic sequencing approaches to identify DNA mutations in cancer are commonly used to predict treatment response and outcome,” says Wang, whose lab studies the molecular mechanisms that regulate leukemia therapy response and survival. “Recent studies, however, show that nongenetic alterations are also key players in drug resistance that cannot be detected by DNA sequencing methods.”
Contributing factors to treatment response
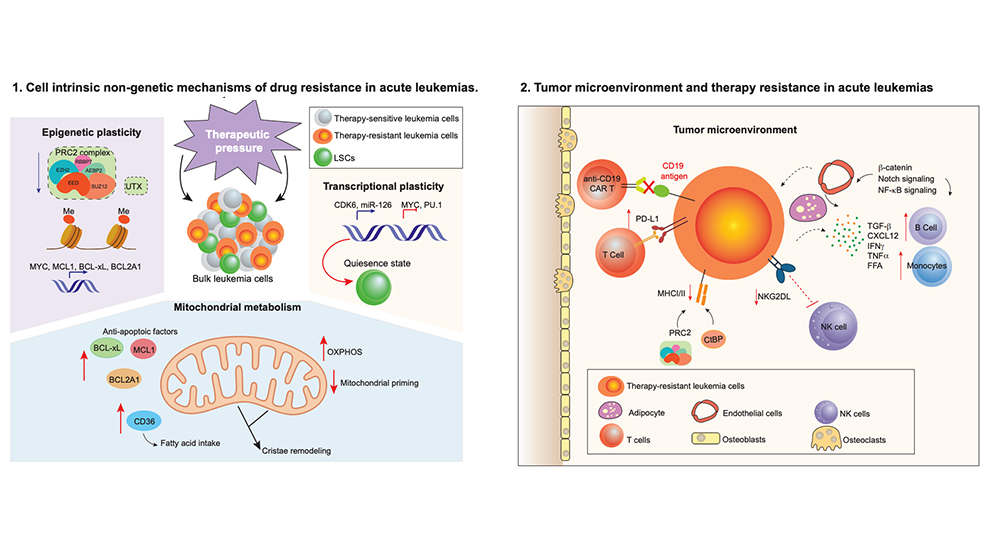
Nongenetic mechanisms of drug resistance include cellular and molecular processes that do not change the underlying DNA code in cells. In their Trends in Cancer review, Wang and his colleagues highlight some of the nongenetic drivers that leukemia cells employ to promote their survival following treatment, including cellular plasticity, dysregulated mitochondria and cell metabolism, and immune-related drug resistance.
Plasticity refers to a cell’s ability to change its physical characteristics in response to external stimuli. Plasticity, the researchers write, enables tumor cells to adapt and survive during exposure to anticancer drugs. For example, a key feature of leukemia stem cells is their ability to enter a reversible state known as cellular quiescence: they become dormant and nondividing when exposed to chemotherapy drugs. Once the drugs are discontinued, the cells resume proliferation and the cancer continues to grow.
Additionally, Wang writes that leukemia cells use epigenetic plasticity, which is when reversible changes occur in cells that are exposed to certain stimuli without altering the underlying DNA code, to avoid the effects of drug therapy. These changes can regulate leukemia stem cell genes associated with chemoresistance. Cellular plasticity and epigenetic mechanisms, therefore, help leukemia cells survive following treatment and may represent a therapeutic vulnerability to overcome drug resistance.
Metabolic changes that drive resistance
Cancer cells’ ability to alter their metabolism to support increased energy requirements for growth, a phenomenon known as metabolic reprogramming, is a hallmark of many cancers, including acute leukemias. Chemoresitant AML cells have high levels of oxidative phosphorylation (OXPHOS), the process by which energy is harnessed through a series of protein complexes embedded in mitochondria, collectively the powerhouse of the cell. Relapsed leukemias often rely on increased nicotinamide metabolism, which can influence many cellular functions, to drive OXPHOS. Studies suggest that these and other metabolic features of acute leukemias play a significant role in treatment resistance.
Influencing the tumor microenvironment
In acute leukemia, the bone marrow microenvironment, the ecosystem of immune and nonimmune cells, blood vessels, and other molecules that surround and support a tumor, can steer malignant cells away from chemotherapy and other treatments. Wang and his team write that nongenetic mechanisms can alter the way leukemia cells are presented to the immune system for destruction by T cells, including nonmalignant cells in the microenvironment that can influence therapy response. Studies have also found that fatty tissue in the microenvironment protects leukemia stem cells from chemotherapy and that atypical B cells enable escape from immune-based therapies.
Overcoming drug resistance
While nongenetic mechanisms of drug resistance remain a challenge, Wang and his colleagues say that all is not lost in the fight against acute leukemia drug resistance. New combination approaches that pair standard leukemia treatments with a new class of drugs that target cancer cells’ pro-survival proteins have shown promise in shutting down the drug resistance that leads to leukemia relapse. Immune checkpoint blockade, which disrupts the protein interactions cancer cells use to evade the immune system, are also being evaluated in clinical trials in combination with more common leukemia treatments. And chimeric antigen receptor (CAR) T-cell therapy, which uses specially altered T cells to attack and destroy cancer cells, has revolutionized treatment for childhood B-cell ALL.
“Understanding nongenetic mechanisms that mediate drug resistance in acute leukemia has the potential to revolutionize patient care by identifying novel biomarkers that correlate with treatment response,” says Wang. “Importantly, this can lead to personalized treatment strategies to prevent relapse and improve long-term survival rates for acute leukemia patients.”