In a recent paper published in The FASEB Journal, Jackson Laboratory (JAX) Senior Director, Innovation and Product Development James Keck, Ph.D., evaluated the effectiveness of a certain type of immunotherapy, bispecific T-cell engagers (BiTEs).
Immunotherapies like BiTEs have revolutionized cancer treatments as they represent a potential off-the-shelf product, yet they are far from perfect. These immuno-oncology based therapies often result in a toxic immune response called cytokine release syndrome (CRS).
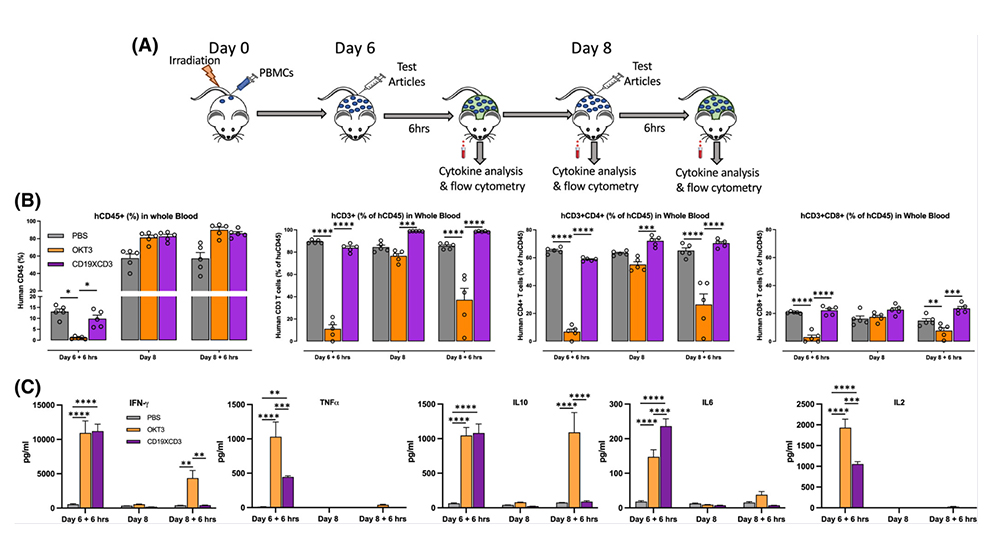
For their work, Keck and colleagues used peripheral blood mononuclear cell engrafted humanized mouse models (PBMC-mouse models) that contain human blood and immune cells to recreate human immune responses. They assessed both the effectiveness and safety of a specific type of BiTE, CD19xCD3 BiTE, also known as blinatumomab, an FDA-approved drug for those who have been diagnosed with B-cell precursor acute lymphoblastic leukemia (B-ALL).
Keck measured tumor burden, activation of immune cells and cytokine release response in several models to identify which was able to reproduce patient response to the therapy. He identified one model that was able to demonstrate both CD19xCD3 BiTE efficiency in controlling tumor growth and the ability to stimulate non-toxic tumor-fighting cytokine release. With further study, this model will allow researchers like Keck to explore the potential of combination therapies involving BiTEs and other drugs to help identify optimal treatment doses and evaluate donor-specific therapeutic windows.