Fibrinogen on the brain: autoimmunity in demyelination diseases
eNews | October 27, 2015Breaching the Blood-Brain-Barrier
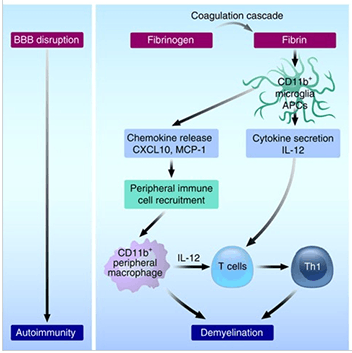
In neurodegenerative conditions such as Multiple Sclerosis and Alzheimer’s disease, disruptions in the blood brain barrier (BBB) allow fibrinogen to contact white matter. Once there, fibrinogen, a non-immunogenic plasma glycoprotein, is converted into and deposited as auto-immunogenic fibrin plaques. The appearance of these fibrin plaques and inflammatory cells that infiltrate the brain after a BBB breach is associated with demyelination and subsequent neuronal dysfunction; however, the mechanism by which this occurs has not been characterized. Recently, Dr. Katerina Akassoglou’s group at The Gladstone Institute of Neurological Disease (University of California, San Francisco) used a chemical approach and a collection of genetically mutant mouse models to elucidate how fibrinogen induces demyelinationin vivo (Ryu et al. 2015).
Consequences of Fibrinogen Leakage into the Central Nervous System
To simulate leaks in the BBB, the Gladstone team injected recombinant fibrinogen directly into the corpus callosum of mice. Remarkably, a single injection of the recombinant fibrinogen was sufficient to induce demyelination. Prior to demyelination, microglia, which are the resident macrophages of the nervous system, and T cells and inflammatory macrophages from the periphery had infiltrated the fibrinogen injection sites. Demyelination was drastically reduced if plasma from fibrinogen-deficient mice or from mice that contained a mutant fibrin that could not bind macrophages was injected. These results suggest that fibrinogen may initiate a neural inflammation-degredation cascade in brain regions with compromised BBB function.
To further understand the contribution of macrophages and T cells in fibrinogen-induced demyelination, several immunodeficient mouse strains were tested for their response to the simulated BBB leakage. In mouse strains defective in macrophage antigen presentation to T cells (MHC Class II null mice, B6.129S2-H2dlAb1-Ea/J (003584); Cxcl10 null mice, B6.129S4-Cxcl10tm1Adl/J (006087)) or lacking T cells (Rag2-deficient mice) demyelination was drastically reduced following fibrinogen injection into the brain. Myelin-specific T-cells harvested from C57BL/6-Tg(Tcra2D2,Tcrb2D2)1Kuch/J (006912) transgenic mice and adoptively transferred into fibrinogen-injected mice both homed to and proliferated in brain regions associated with the injection, whereas ovalbumin-specific, control T-cells harvested from OT-II (B6.Cg-Tg(TcraTcrb)425Cbn/J (004194)) transgenic mice did not. Finally, analysis of the corpora callosa collected from fibrinogen-injected mice revealed an inflammatory Th1 gene signature, suggesting that fibrinogen induces activation of cytotoxic T cells.
Collectively, these data indicate that fibrinogen, a beneficial product of the blood coagulation cascade in the circulation, stimulates neuroinflammation and subsequent neural dymyelination and dysfunction in the central nervous system. Further, this model of induced neuroinflammation represents a novel and direct method to assess the biological consequences of a disrupted blood-brain-barrier, which occurs in a variety of neurological diseases.
Additional JAX mice used in this research paper, but not specifically described above:
B6.129S7-Rag1tm1Mom/J, 002216 B6.129S4-Itgamtm1Myd/J, 003991 B6.129(Cg)-Ccr2tm2.1Ifc/J, 017586 B6.129P-Cx3cr1tm1Litt/J, 005582 SJL/J, 000686
