Novel inhibitor targets vascular and cognitive defects in AD mice
October 28, 2014Mounting evidence indicates that cerebrovascular abnormalities are significantly involved in the development of Alzheimer’s disease (AD). A majority of dementia patients display both AD and cerebrovascular pathologies, including altered cerebral blood flow and microinfarcts, demonstrating a critical link between vascular risk factors and AD pathology. Recent evidence points to fibrinogen as the link between the neurological and vascular defects in AD patients. Fibrinogen is the primary protein component of blood clots, but is normally prevented from entering the brain through the blood-brain barrier. However in AD patients, fibrinogen is found in brain blood vessels and parenchyma. Additionally, β-amyloid (Aβ), the main component of amyloid plaques, binds to fibrinogen and alters fibrin clot structure, making them resistant to degradation. Therefore, molecules that can block Aβ-fibrinogen interaction without affecting normal thrombosis, or blood clots, are strong targets for AD therapeutics. A new report inJ. Exp. Med. (Ahn et al. 2014) identifies a novel inhibitor of Aβ-fibrinogen interaction that dramatically improved both vascular defects and cognitive impairment in a mouse model of AD.
High-throughput screen to identify a novel Aβ-fibrinogen interaction inhibitor
High-throughput screening of >93,000 small molecules identified 10 candidate compounds that inhibit Aβ-fibrinogen interactions. A focused analogue library of >2,000 compounds derived from these primary drug candidates further identified five compounds with improved Aβ-fibrinogen inhibitory activity. One of these five molecules, RU-505, displayed the greatest inhibitory efficacy. Aβ molecules can aggregate to form oligomers, which are believed to be the principal agent of nerve cell death in AD. Aβ42 monomers and oligomers both interact with fibrinogen, but the Aβ42 oligomers have a ~4 fold greater affinity than the monomers. RU-505 inhibits interaction between fibrinogen and both Aβ42 monomers and oligomersin vitro, but the inhibition efficacy is greater against Aβ42 monomer-fibrinogen interactions. Additionally, RU-505 significantly reduced Aβ42-induced changes in fibrin clot formation and clot degradation in vitro, without affecting fibrin clot formation and degradation in the absence of Aβ42. Therefore, RU-505 inhibits only the interaction between fibrinogen and Aβ without altering basal thrombosis. 5XFAD mice treated for 4 months with RU-505 displayed a significant (~37%) reduction in occluded vessels following FeCl3-induced cerebral thrombosis as compared to vehicle treated mice. RU-505 administration did not affect basal vessel occlusion in wild type (WT) littermates.
RU-505 ameliorates the altered thrombosis in AD mice
The B6SJL-Tg(APPSwFlLon,PSEN1*M146L*L286V)6799Vas/Mmjax (006554, aka 5XFAD or Tg6799) mice express mutant human APP with three familial Alzheimer's disease (FAD) mutations and human PSEN1 harboring two FAD mutations in the brain. 5XFAD mice recapitulate major features of AD, including amyloid plaque formation starting at 2 months of age, neuron and synaptic loss, and cognitive deficits. 5XFAD mice treated for 4 months with RU-505 displayed a significant (~37%) reduction in occluded vessels following FeCl3-induced cerebral thrombosis as compared to vehicle-treated mice. RU-505 administration did not affect basal vessel occlusion in wild type (WT) littermates.
Aβ-fibrinogen interaction reduces vascular Aβ deposition and cognitive impairment
In addition to ameliorating the abnormal thrombosis in the 5XFAD mice, long-term RU-505 administration significantly:
- Decreased β-amyloid deposition in cerebral blood vessels
- Reduced infiltrated fibrinogen and microgliosis in the cortex
- Improved cognitive deficits
These exciting results strongly demonstrate that targeting the interaction between Aβ and fibrinogen is an effective method for improving the cerebrovascular and cognitive defects in an AD mouse model, and offers a novel target for new AD therapeutics.
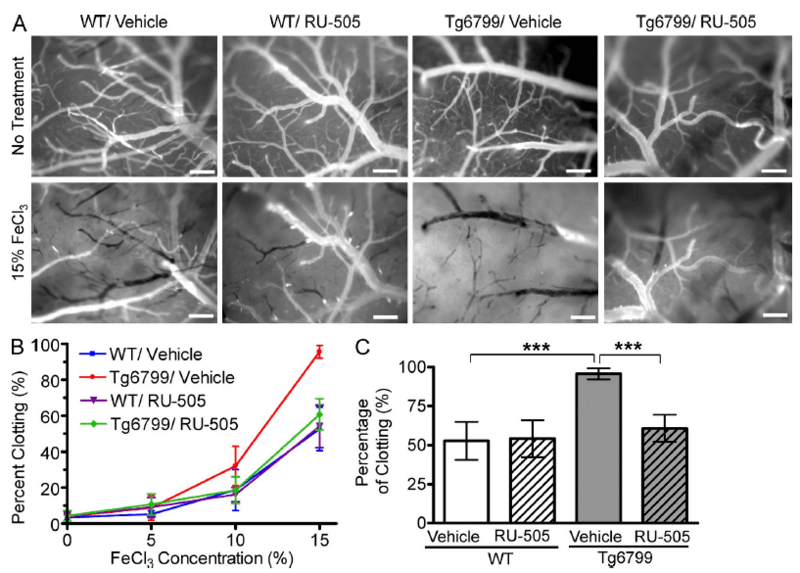
RU-505 prevented altered thrombosis and fibrinolysis in AD transgenic mice
(A) After craniotomy, three concentrations of FeCl3 (5, 10, and 15%) were incrementally administered to the surface of the brains of vehicle- or RU-505–treated WT and Tg6799 (5XFAD) mice, and clotting of cerebral blood vessels (>20 µM) was imaged (bars, 200 µm). Representative images show the surface of the brains of vehicle- or RU-505–treated WT and 5XFAD mice before FeCl3 treatments or 5 min after 15% FeCl3 treatments. (B and C) Frequency of clotted vessels was calculated at increasing concentrations of FeCl3 (B) and was plotted for 15% FeCl3 treatment (C; ***, P < 0.001; n = 5 mice per group). All values are means and SEM. Results are from two independent experiments.
