Understanding the tumor-stroma relationship
Stromal cells found within epithelial tumors can have a significant impact on the growth, vascularization, invasiveness and metastatic potential of the cancer. These features make stromal cell populations potential therapeutic targets. A new publication in the journal Digestive Diseases and Sciences (Maykel et al., 2014) demonstrates the importance of immunodeficient host mouse selection for efficient propagation of primary human tumors referred to as patient-derived xenografts (PDX), maintenance of tumor microenvironment, and in establishing a platform for in vivo therapy testing.
NSG and NRG mice are a superior host for PDX
For many years, NOD scid (NOD.CB17-Prkdcscid/J, 001303) and nude (NU/J, 002019 or J:NU, 007850) mice have commonly been used as hosts for cancer cell lines in basic and therapeutic research. Researchers now recognize that therapies developed using these homogeneous cell lines, which are devoid of stroma, translate poorly to the clinic. Current focus is now shifting toward heterogeneous, primary human tumors. The functional innate immunity (NK cells in particular) of these immunodeficient hosts, however, is a barrier to engraftment and maintenance of tumor architecture for many types of primary human cancers. Two newer mouse strains overcome this challenge: NSG (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ, 005557) and NRG (NOD.Cg-Rag1tm1Mom Il2rgtm1Wjl /SzJ, 007799).
The interleukin-2 gamma chain(gene symbol: Il2rg) is a common subunit of the receptors for IL-2, IL-4, IL-7, IL-9, IL-15 and IL-21. The Il2rg null mutation in NSG and NRG mice prevents functional development of NK cells, allowing engraftment of a wide range of human PDX. NSG mice are deficient in DNA repair following V(D)J recombination due to the Prkdcscid mutation. Similarly, NRG are deficient for Rag1 (recombination activating gene 1), which encodes the enzyme that directly mediates V(D)J recombination. Either mutation prevents development of functional B and T cells.
Maykel et al. obtained primary human colon cancer samples from multiple donors and compared tumor take rates and growth characteristics in NOD scid, NSG, and NRG hosts. Tumor-take rates in NOD scid mice were 46%, while those in NSG and NRG mice were 90% and 91%, respectively. (Ten different primary donor samples were tested in each host, and 4-12 mice were included in each test group.) Additional advantages of the NSG and NRG mice over NOD scid included:
- Faster growth of palpable tumors
- More consistent and uniform growth between recipients
- Improved maintenance of tumor architecture
- Reduced tumor infiltration by host neutrophils
- Maintenance of gland structure and blood vessel
Stroma turnover in colon PDX
Immunohistochemical evaluation of tumors grown subcutaneously in NSG and NRG mice demonstrated that human colon cancer epithelial cells remained highly proliferative. In contrast, human tumor stromal cells showed little to no evidence of proliferation. Staining of tissue sections for the human stroma marker vimentin revealed a steady decrease of stromal cell numbers by 4 weeks post-engraftment in the primary host and a total loss of human stroma in secondary recipients. “Passenger” human leukocytes that accompanied the primary tumor grafts were lost in a similar pattern.
The overall maintenance of tumor growth and structural architecture suggested that the human stromal cells were dynamically replaced by mouse-derived stroma. This question was addressed by first propagating the tumors in NSG mice followed by re-engrafting the tumors into NSG-GFP (NOD.Cg-Prkdcscid Il2rgtm1Wjl Tg(CAG-EGFP)1Osb /SzJ, 021937) secondary recipients. The NSG-GFP hosts express green fluorescent protein in most cells and tissues. Immunohistochemistry and direct fluorescence confirmed that mouse-derived stromal cells enter the human colon tumors following re-engraftment into the NSG -GFP secondary hosts. Therefore, human tumor-derived stromal fibroblasts are slowly lost during PDX tumor expansion in mice and concomitantly replaced by mouse-derived stromal fibroblasts (see figure 1).
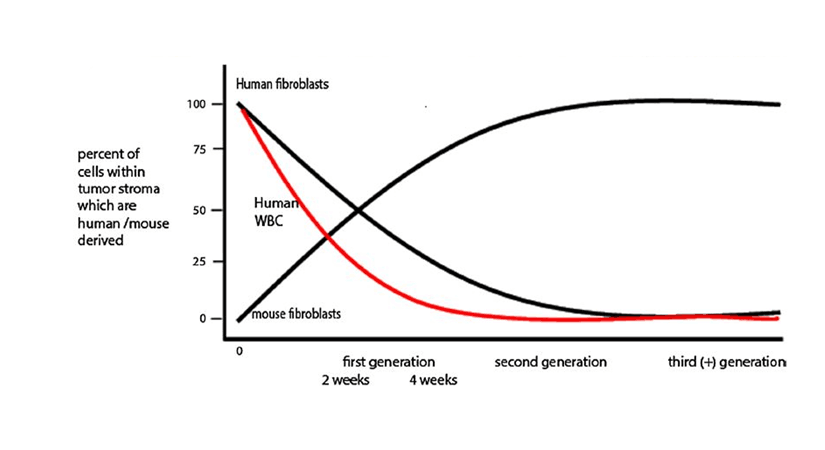
Figure 1. Schematic representation of stromal cell turnover in human colon cancer expanded in the NSG mouse model. Reproduced with permission from Digestive Diseases and Sciences.
Colon tumor response to chemotherapy
Both the NSG and NRG bearing colon PDX were tested for responsiveness to 5-fluorouracil (5-FU) chemotherapy. The host mice maintained normal hemoglobin and hematocrit 14 days after the start of 5-FU therapy, but, as expected, showed a drop in white blood cells (WBC) count. Tumors showed disrupted cell organization, decreased cell viability, blood vessel loss, necrosis and mouse leukocyte infiltration. These data provided evidence indicating that both NSG and NRG hosts are suitable platforms to propagate patient-derived tumors and to test new treatment strategies.
What’s next?
The research presented by Maykel et al. establishes the utility of three different mouse strains (NSG, NRG, and NSG-GFP) in examining the relationship between stromal fibroblasts and tumor growth. Tumor stroma is known to play an important role in tumor growth (Li, Fan, and Houghton, 2007) and the strains evaluated in this study enable closer examination of the biology of these cellular interactions. This research is important because it raises awareness to investigators that the stromal cells within PDX tumor models can change over time and passage. It remains unclear how these changes might impact tumor gene expression profiles or the validity of preclinical efficacy studies. The stromal changes, however, provide a unique opportunity to understand how human versus mouse stroma influence tumor growth and may lead to identification of new targeted therapies to modify tumor growth and survival.
Overall, PDX-engrafted NSG and NRG host animals provide an improved preclinical testing platform to test new therapies and allow for the molecular characterization of the tumors to enable custom-designed combinatorial therapies. These “personalized” therapies then could be tested for efficacy in these PDX-engrafted mice prior to clinical implementation to improve both safety and efficacy.