JAX offers preclinical imiquimod-induced psoriasis mouse models as a rapid screening approach for candidate psoriatic therapies.
JAX offers preclinical imiquimod-induced psoriasis mouse models as a rapid screening approach for candidate psoriatic therapies.
Imiquimod (IMQ) is a toll-like receptor agonist that acts as an immune response modifier. Topical application of IMQ to the skin of susceptible BALB/cJ and C57BL/6J mice induces inflammation with features commonly found in human psoriatic skin, including erythema and scaling. The advantage of this model over other inducible psoriasis models is the fast induction of clinical phenotypes for rapid therapeutic screening.
Phenotype | BALB/cJ(JR# 000651) | C57BL/6J(JR# 000664) | Method |
|---|---|---|---|
Clinical Observations | ✓ | ✓ | PASI Scoring & Digital Images |
Skin Histopathology | ✓ | ✓ | H&E Staining and Semi-Quantitative Scoring |
Phenotyping Skin Resident Lymphocytes | ✓ | ✓ | Flow Cytometry |
PBMC/Spleen/Lymph Node Phenotyping | ✓ | ✓ | Flow Cytometry |
Serum Cytokines | ✓ | ✓ | Meso Scale Discovery |

Contact Us
[email protected]
1.800.422.6423 (US)
1.207.288.5845 (International)
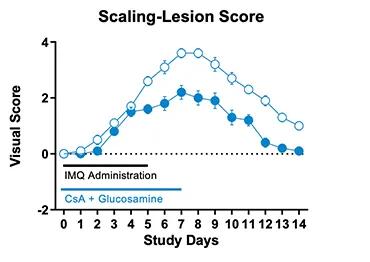
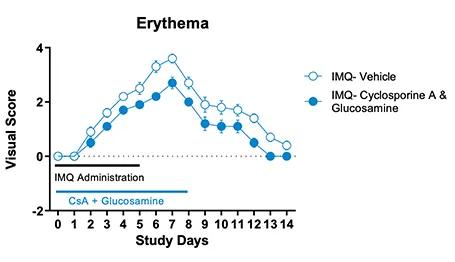
Figure 1. IMQ-induced skin psoriasis and inflammation in mice. BALB/cJ mice were treated daily for 6 days with IMQ cream of control (petroleum jelly) on the shaved back skin and received either cyclosporine A + glucosamine or vehicle. Cyclosporine A + glucosamine reduced the Scaling and Lesion severity score as well as the Erythema score compared to vehicle-treated mice.
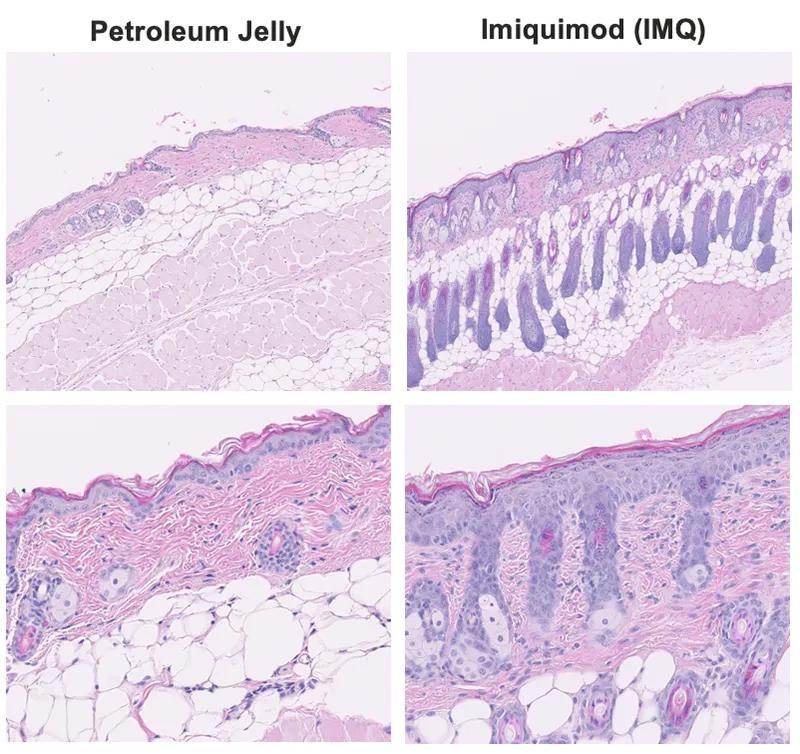
Figure 2. Histopathological evaluation in skin from Imiquimod-induced psoriasis. H&E staining showed that IMQ applied to the skin of BALBc/J mice resulted in vasodilation, infiltration of inflammatory cells in epidermis and dermis, increased epidermal thickening, and increased keratinocyte differentiation compared with the control (petroleum jelly-treated group).