To facilitate strain importation and microinjection experiments, many JAX® Mice are readily available as frozen embryos and/or sperm. Custom, made-to-order embryos are available upon request for strains not readily available. Standardized protocols for handling and media preparation are provided below to support successful live mouse IVF propagation at your facility.
Key Benefits:


Proven Quality & Reliability
All sperm and embryos are collected and cryopreserved in our highest-barrier facility. Embryos undergo multiple washes to ensure they arrive clean and ready for your research.
Strain Fidelity
JAX maintains precise genetic backgrounds, especially for complex or proprietary strains (like NSG mice), ensuring consistency across studies and institutions.

Scalability & Support
JAX offers scalable supply and customer support to facilitate the success of recovery even in tight timelines.
For frozen embryos, each straw or vial consists of approximately 30 embryos at the 2-cell or 8-cell stage. Enough embryos are provided to recover the genotype of interest. When cryorecovery is performed by JAX, JAX’s standardized protocols are sufficient to recover the strain.*
For frozen sperm, JAX’s standard offering includes one straw per strain. When IVF is performed by JAX using the protocols below, this is sufficient to result in at least one litter of mice.*
*JAX makes no guarantee concerning embryo transfer or fertilization success, sex ratios, or genotype ratios when performed outside JAX facilities.
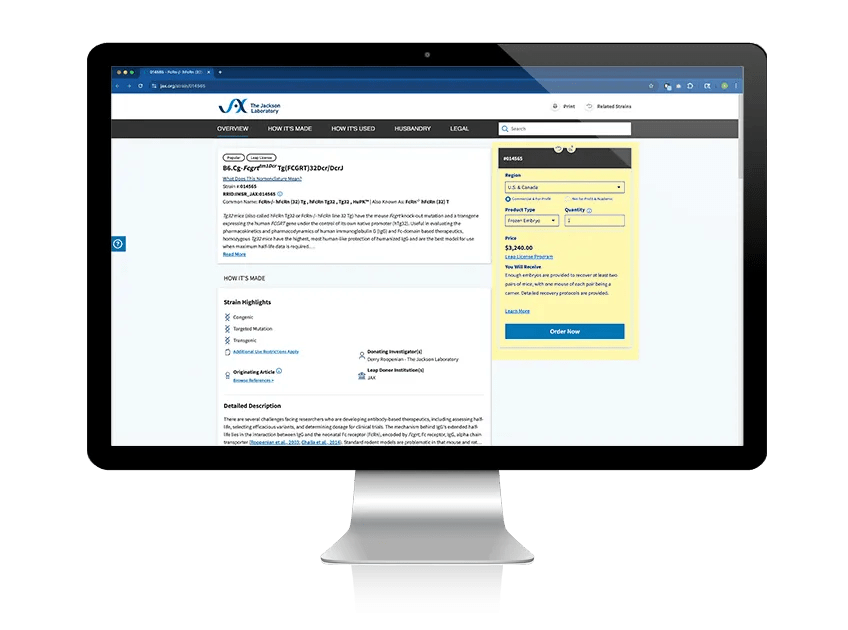
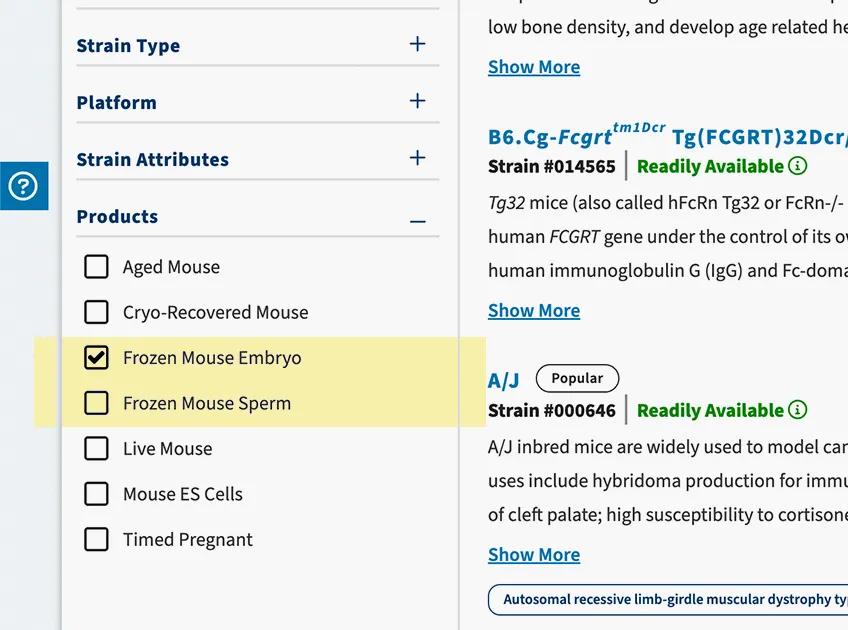
Search for available strains
Use JAX's Mouse Search tool to filter for strains available as frozen embryos or sperm. If your strain of interest is not listed, contact us to talk about custom made-to-order options.
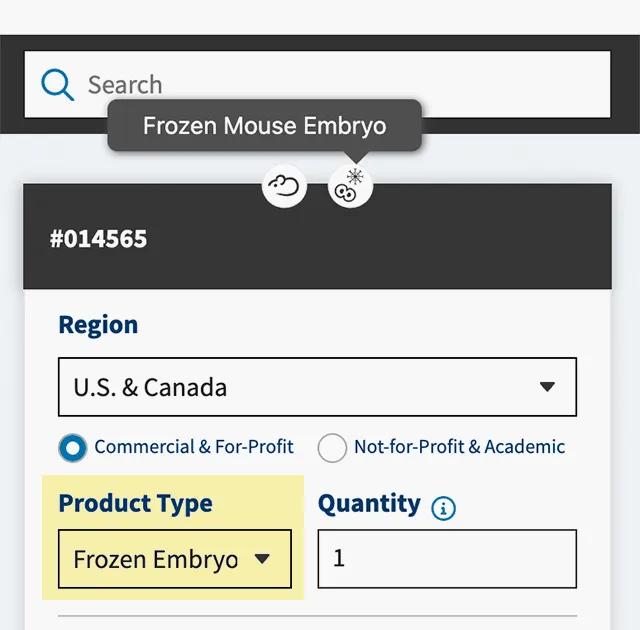
Select the 'Frozen Embryo' or 'Frozen Sperm' product type
Using the "Product Type" drop-down menu on the strain datasheet, select the "Frozen Embryo" or “Frozen Sperm” item and enter the quantity of straws needed.
Place your order
Place your order using the same order form you use when ordering JAX™ Mice.
Recovery Thaw Protocol for Straws
Recovery Thaw Protocol for Vials
JAX Cryopreservation Media Preparation
JAX scientists have conducted extensive research in mouse strain cryopreservation techniques to establish highly refined cryoprotectant solutions and optimal protocols for preserving models on diverse genetic backgrounds. These developments are the basis for affordable conservation and colony management practices implemented at JAX, as well as our ability to maintain and distribute more than 11,000 genetically modified mouse lines around the world. Read on to find the answers to our most common questions regarding the cryopreservation, cryorecovery, and breeding services offered at JAX.
Not what you're looking for?
Visit our Resource Hub