By Jens Rueter, MD and Andrey Antov, PhD, MBA
On behalf of all of the participating oncology practices in Maine, we are happy to share the latest progress updates for the Maine Cancer Genomics Initiative. To date, 100 patients have been registered and 58 clinicians at 10 sites are participating in the Study. The MCGI Genomic Tumor Board is an example of the Molecular Tumor Boards being put into place across the oncology field to better support interpretation of cancer genomic testing results. MCGI Genomic Tumor Board Advisors, Ben Park MD PhD and Josh Lauring, MD PhD spoke to NPR this month about targeted therapies. To read the article, click here.
The MCGI has scheduled more than 30 Genomic Tumor Boards sessions so far for 2018. Feel free to reach out to us at [email protected] for full list of GTB dates this year and details on CME offered for participants.
February:
To learn more about MCGI, please visit our updated website at www.jax.org/mcgi, including biographies of Clinical Steering Committee members and our External Advisors. The Initiative is thankful to our External Advisors and Steering Committee members for all they do to support the Initiative. We are also thankful for the many Maine oncologists, clinicians, and research staff participating in the Study. In each newsletter we will feature a key MCGI community member in this Spotlight.
This month we feature Dr. Christian Thomas. Dr. Thomas is both an MCGI Steering Committee member and the Principal Investigator for the MCGI Study at New England Cancer Specialists.

Christian Thomas, MD
Dr. Thomas joined New England Cancer Specialists as a physician and the Director of Clinical Research in 2012. His clinical focus is on thoracic cancers (lung cancer, esophageal cancer) as well as GU cancers (prostate, testicular, bladder and kidney cancers). He also serves as an advisor to the American Society of Clinical Oncology, the Northern New England Clinical Oncology Society and CMS/Medicare. Dr. Thomas completed his medical school training in Frankfurt, Germany and an Internal Medicine residency and Hematology/Oncology fellowship at Colombia University in New York City.
By Gregory Lewis, PharmD, RPh, Clinical Genomic Scientist
The JAX Action-Seq™ report contains a wealth of information about a patient’s tumor testing results. Over the course of several newsletters, we will highlight different aspects of the report and provide details that can help in interpreting the information provided. In this issue we present:
CLIA Educational Brief – Overview of Clinical Interpretation
All ActionSeq Plus reports contain summaries for the targeted drugs included based on interpretive findings. Both approved and investigational drug descriptions cover fundamental pharmaceutical information such as the USAN generic name, brand name, drug class and mechanism of action in addition to an overview of the clinical evidence associating the drug with the genomic alteration. FDA approved therapies also contain the current approval status and prescribing indications. Additionally, ClinicalTrials.gov is reviewed and targeted clinical trials that potentially provide therapeutic avenues for the patient based on their genomic profile and pathology report are also included. Evidence supporting the role of these therapeutic findings in cancer are collected from FDA.gov, NCCN clinical practice guidelines, peer-reviewed literature, public databases and JAX-CKB. Based on this information, variants with molecular classifications are clinically classified according to the joint AMP/ASCO/CAP variant interpretation guidelines1. Due to the rapid pace of oncology treatment research, comprehensive re-assessment of gene-drug response is conducted on a 6-month cycle to ensure the most accurate and current information is provided in the clinical report.
Figure 1: Components of therapeutic information

1Marilyn M. et al., Standards and Guidelines for the Interpretation and Reporting of Sequence Variants in Cancer: A Joint Consensus Recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists Mol Diag, Vol 19, 2017, P. 4-23, ISSN 1525-1578, https://doi.org/10.1016/j.jmoldx.2016.10.002
As always; Information about test processing status is available by contacting Shannon Rowe in Clinical Lab Customer Service, [email protected]
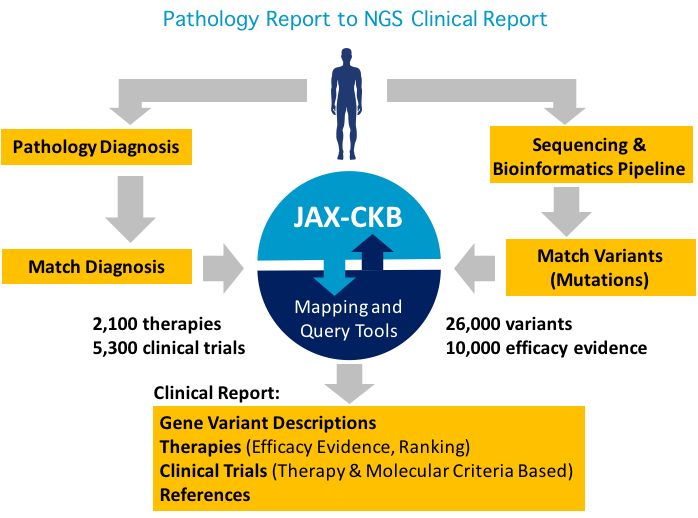
The JAX Clinical Knowledgebase is a publicly available resource for supporting cancer variant interpretation. It is well utilized in the scientific community and has recently been adopted by a number of clinical laboratories outside of JAX to support variant interpretation.
The Jackson Laboratory Clinical Knowledgebase (JAX-CKB):
By Susan Mockus, PhD and Kunal Sanghavi, MBBS, MS, CGC
Personalized medicine has undeniably had the greatest impact to oncology and while the understanding and treatment of cancer patients has improved, data interpretation still remains a significant bottleneck. JAX-CKB (jax.org/ckb) has over 26,000 gene variants and informs patient cancer care while contributing to technology and methodology development to advance the field of oncology precision medicine. JAX-CKB user survey results were recently presented at theAmerican Society for Human Genetics (ASHG) annual meeting in Orlando, FL.
Approximately 94% of respondents indicated they would refer a colleague to CKB.
The 2018 MCGI Forum will be held on Fri April 6th through Sun April 8th at the Samoset Resort in Rockport, ME. Attendance is by invitation only. Lodging and meal costs will be covered for invited Maine clinicians and research personnel. Invitees may bring a spouse or guest; nominal meal charges will be assessed at registration for guests of invitees.
The group will have complimentary access to unlimited local and toll free calls, in room coffee, wireless internet in guest rooms and public space, access to the business center, outdoor zero entry pool, hot tub and fire pit, outdoor recreation including tennis courts, shuffleboard, basketball court and playground, concierge service, children’s activities, and access to our state of the art full service health club including indoor pool, hot tub, steam saunas, strength room, group fitness room, cardio theater, and classes.
Jan 2nd Jens Rueter, MD and Andrey Antov, PhD, MBA presented a status review of the Maine Cancer Genomics Initiative at the MaineGeneral Hospital Board of Directors meeting.
Jan 24th: Jens Rueter, MD and Andrey Antov, PhD, MBA presented an overview of the Maine Cancer Genomics Initiative at a lunchtime seminar at the Maine Medical Association.
___________________________________________________________________________________________________________________
The mission of the MCGI is to enable widespread access to clinical cancer genomic tests for the Maine oncology community and to increase the understanding of cancer genomics by Maine oncology clinicians. The Maine Cancer Genomics Initiative (MCGI), enabled through the generous financial support from The Harold Alfond® Foundation, leverages the strengths of key medical and bioscience research institutions in Maine to create and alliance focused on precision cancer diagnostics and treatment.
The MCGI central office team is dedicated to ensuring your practice’s engagement and experience with genomic cancer test-ing. Please feel free to reach out with any questions or comments. Email us at [email protected]