The functional mechanisms that may underlie ME/CFS
Research Highlight | February 7, 2023
Those with the disease have been — and still often are — dismissed as overreporting symptoms, or told that their physical dysfunction is psychosomatic, arising from mental health issues.
Jackson Laboratory (JAX) Professor Derya Unutmaz, M.D., and Associate Professor Julia Oh, Ph.D., have teamed with ME/CFS physicians and other experts to help remove the obstacles. Supported by the National Institutes of Health (NIH) as one of three ME/CFS Collaborative Research Centers, they have been investigating what current tests don’t reveal: what’s different in ME/CFS patients that, though difficult to find, causes such debilitating disease? Their studies have involved exhaustive analyses of immune cells and metabolites in their blood, and the microbes in their bodies. Does dysfunction in the immune system, metabolism or microbiome play the key role, or are they interrelated? And are there clear differences between ME/CFS patients and healthy controls that can lead to efficient diagnoses and help inform subsequent care?
Microbes and metabolism
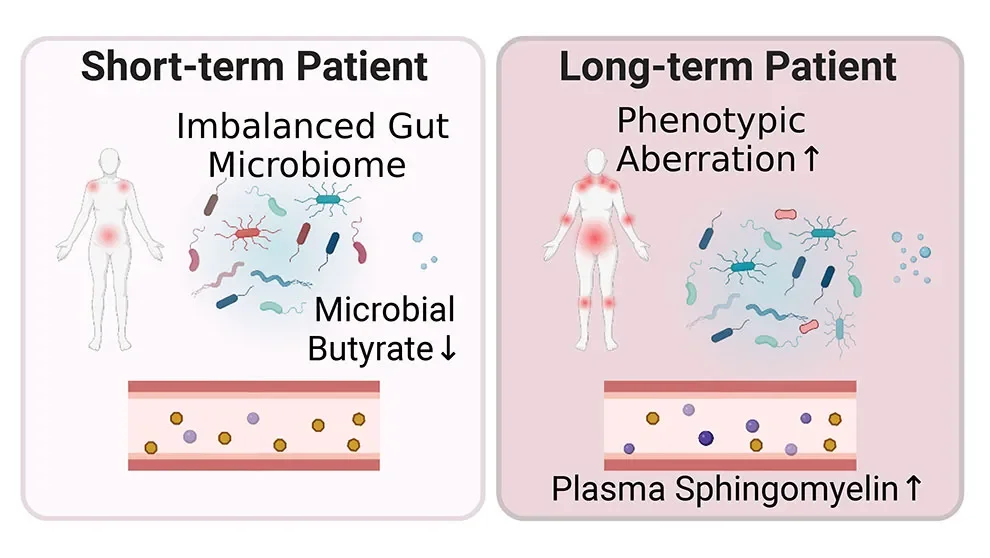
A new study from Oh, Unutmaz and their collaborators delves into host-microbiome interactions in ME/CFS as well as potential metabolic consequences. Published in Cell Host & Microbe, "Multi-'omics of host-microbiome interactions in short- and long-term Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS)" looks at individuals with ME/CFS divided into two groups — those diagnosed less than four years ago or more than ten — as well as matched healthy controls. In addition to blood and stool samples, the researchers collected extensive clinical and lifestyle data from the participants. What they found were microbial and metabolic traits that differed between the groups, including multiple biomarkers that were specific to the individuals with ME/CFS.
"The microbiome has emerged as a potential contributor to ME/CFS," says Vicky Whittemore, Ph.D., program director at NIH’s National Institute of Neurological Disorders and Stroke (NINDS). "These findings from the NIH-funded ME/CFS Collaborative Research Center at JAX provide unique insights into the role of the microbiome in the disease and suggest that certain differences in gut microbes could serve as biomarkers for ME/CFS."
Previous research has indicated that altered gut microbiota may underlie the gastrointestinal problems associated with ME/CFS. The studies were relatively small, however, and had limited resolution. Oh, Unutmaz and the team therefore performed extensive, high-resolution analyses of the gut microbiomes of the study participants. Overall, they found that samples from people with ME/CFS had more uneven but less diverse microbial communities, with a modest but broad dysbiosis similar to that seen with aging and chronic inflammatory disorders. Interestingly, however, when the researchers looked at the short- and long-term ME/CFS groups separately, they found that the short-term group had the most significant gut microbiome disruption. The long-term cohort had returned to a state closer to that of the controls, showing more normal microbial diversity with the reacquisition of some low abundance species. Nonetheless, the long-term group had the more severe disease-associated traits, including fibromyalgia and worsening sleep problems, as well as metabolic abnormalities.
Biomarkers for ME/CFS
Looking further, the team found that low abundance microbes implicated in tryptophan, butyrate and propionic acid production were largely absent in individuals with ME/CFS. These substances are important for regulating metabolic and endocrine functions, including modulating inflammatory responses. Given the critical roles butyrate plays in intestinal cells as a major energy source and an anti-inflammatory, the researchers focused further on the butyrate pathway to better understand the role it might play in ME/CFS. They found that plasma isobutyrate is depleted in those with ME/CFS, and that the microbiome data also predicts lower butyrate abundance and changes in the ability of the gut microbiome to metabolize or synthesize short-chain fatty acids. Overall, the pathway affects dozens of plasma metabolites, and its disruption can have both metabolic and immunologic consequences.
To help with potentially diagnosing ME/CFS cases, Oh and Unutmaz constructed multiple classifiers involving two microbiome attributes, metabolite abundance, and a combination of the three models. The combination, termed multi-'omics, performed better than any individual set of data in differentiating individuals with ME/CFS from healthy controls. The depletion of the low abundance microbes referenced above was an important discriminatory feature, as well as levels of blood metabolites such as betaine. These features point to ways to increase classification accuracy and the development of novel therapeutic strategies.
"Indeed, future studies that include diseases with overlapping traits and symptoms, such as long COVID or fibromyalgia, would be powerful for enhancing this classifier such that it may have promise to aid in diagnosis and differentiation of this often-misclassified disease," says Oh.
Viral trigger?
The study establishes a framework through which to study host-microbiome interactions and glean better understanding of the mechanisms underlying ME/CFS. The authors also note that research into ME/CFS has recently taken on further urgency. It has been conjectured that viral infections may trigger the onset of ME/CFS, and they were frequently reported in the ME/CFS groups in the lifestyle/behavioral survey component of the study. The recent COVID-19 pandemic and the emergence of "long COVID" following initial SARS-CoV-2 infection may be associated, as many long COVID patients share symptoms with people with ME/CFS, including lingering fatigue and myalgias. Progress in either long COVID or ME/CFS research may therefore provide diagnostic and therapeutic insight for both conditions.
The study was supported by NINDS grant U54NS105539.

