Superagonizing NK cells help fight HIV
eNews | October 14, 2015Activation of NK cells to fight HIV infection
Natural killer (NK) cells, the “rapid response” cells of the innate immune system, can kill virally infected cells and thereby slow down an infection until antigen-specific and clonally-expanded cytotoxic T cells can be recruited to finish the job. In a new study published in theJournal of Virology (Seay et al., 2015), investigators at the Albert Einstein College of Medicine in Bronx, NY, used an engineered IL-15 superagonist to activate human NK cells inhu-PBMC NSG™ humanized mice and prevent the spread of HIV infection. The study provides compelling, preclinical evidence for using this approach to treat acute HIV infection.
IL-15 superagonist schematic. IgG1 Fc domains are fused to each of the two IL-15Rα 65 amino acid Suchi domains. The opposite ends of the IL-15Rα molecules are bound to a genetically modified IL-15 containing asparagine converted to aspartic acid at position 72 (N72D). This amino acid substitution improves the modified IL-15’s binding affinity to IL-15Rα. The IL-15 superagonist has a 25-fold greater activity and >35 fold longer half-life than the native IL-15::IL-15Rα complex.
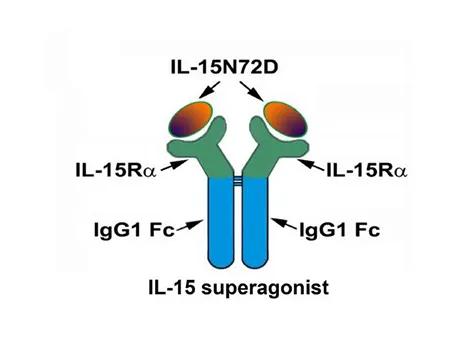
IgG1 Fc domains are fused to each of the two IL-15Rα 65 amino acid Suchi domains. The opposite ends of the IL-15Rα molecules are bound to a genetically modified IL-15 containing asparagine converted to aspartic acid at position 72 (N72D). This amino acid substitution improves the modified IL-15’s binding affinity to IL-15Rα. The IL-15 superagonist has a 25-fold greater activity and >35 fold longer half-life than the native IL-15::IL-15Rα complex.
Harnessing the killing power of NK cells
NK cells are activated by IL-15 through a multi-step pathway. Activated dendritic cells and macrophages at an infection site make IL-15 that binds IL-15Rα, forming a membrane bound complex. The IL-15::IL-15Rα complex binds and activates adjacent NK and CD8+ T cells through IL-15Rβ and IL-15Rγ. The IL-15::IL-15Rα complex also can be released into serum, where it remains stable and retains activity. Seay and colleagues created an IL-15 superagonist with a 25-fold greater activity and 35-fold longer half-life than the native IL-15::IL-15Rα complex (see figure). They demonstrated functional NK cell activation using three assays. First, after culturing human peripheral blood mononuclear cells (hu-PBMCs) with the IL-15 superagonist, flow cytometry revealed increases in granzyme B and perforin expression in the CD3-, CD16+, CD56+ NK cells. Second, when hu-PBMCs were treated with the IL-15 superagonist in vitro and subsequently incubated with K562 cells, a well-characterized NK target cell population, the treated NK cells showed increased Cd69 and CD107a expression, markers for activation and degranulation, respectively, compared to untreated hu-PBMCs. K563 cell lysis in the treated hu-PBMC co-cultures was observed, also. Finally, IL-15 superagonist-treated hu-PBMCs could lyse ACH2 cells, a latent HIV-infected cell line that produces active virus following stimulation with phorbal ester (PMA) or TNF-α. Together, these experiments demonstrate that the IL-15 superagonist can activate human NK cells and kill HIV-infected cells in vitro.
Validating the IL-15 superagonist in HIV infected humanized mice
Validating the IL-15 superagonist in HIV infected humanized mice
To further their studies, the Einstein investigators developed a unique approach to modeling HIV infection in mice. Using the highly immunodeficient NSG™ mice [NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (005557)] as a host, they injected hu-PBMCs directly into the spleen along with HIV engineered to express luciferase (HIV-LucR). Luciferase has a short half-life (~3 hours), allowing its expression to be a quantitative measure for active viral replication. Luciferase expression can be quantified both in cell lysates and in live mice using a luminometer.
In an initial in vivo experiment, hu-PBMC NSG™ mice were infected with HIV-LucR and one day later were treated with the IL-15 superagonist. Five days later, the live, treated mice showed a significant reduction in total luminescent flux, and spleen lysates from them showed a 99% reduction in luciferase expression compared to HIV-LucR infected but untreated controls. Flow cytometry revealed no significant changes in NK cells and CD8+ T cell frequencies in the spleens of the treated mice, suggesting that activation of existing cells was responsible for killing the HIV-LucR-infected cells. The Einstein team also observed a three-fold increase in the activation marker CD69 in the human CD3-, CD16+, CD56+ NK cells harvested from the spleens of the IL-15 superagonist-treated mice, suggesting that activated NK cells controlled the acute HIV infection.
Finally, in a third in vivo experiment, the Einstein team delayed treating the HIV-LucR-infected hu-PBMC NSG™ mice with IL-15 superagonist until 5 days post-infection. As before, luciferase activity in these animals’ spleens was reduced by >90% one day after treatment. When spleens from infected mice were examined for sustained control of infection at later time points, however, luciferase expression had rebounded. These data indicate that once an HIV infection was established, the IL-15 superagonist could not activate NK cells to control the infection.
To establish that the IL-15 superagonist’s effect on HIV-infected cells is acting primarily through NK cells, the authors performed a cell depletion study: prior to intrasplenic injection, the hu-PBMCs were depleted of either NK cells or CD8+ T cells. When compared to unfractionated hu-PBMCs, the CD8+-depleted hu-PBMC recipients show similar reductions in luciferase expression following IL-15 superagonist treatment. In contrast, recipients of NK cell- depleted hu-PBMC fractions were not able to clear HIV-LucR. Together, these data clearly demonstrate that the activated NK cells were responsible for killing and clearing the HIV-infected cells.
The data presented in the Einstein study demonstrates that their IL-15 superagonist can activate human NK cells and that these activated cells can prevent an HIV infection from progressing by recognizing and killing HIV-infected target cells. NK cells are one of the innate immune systems “first responders” that help to control an infection while the adaptive immune responses are gearing up. The approach described here demonstrates that once these NK cells are activated, they can robustly control an acute HIV infection. The hu-PBMC NSG™ model provides a useful preclinical testing platform to further identify new treatment strategies for eradicating of both acute and chronic HIV infections.
