Interleukin-10 gene targeted mutation
JAX Notes | October 1, 1997Interleukin-10
Interleukin-10 (IL-10) is an important regulatory cytokine that suppresses effector functions of macrophages/monocytes, T helper 1 (Th1) cells, and natural killer cells (for a review, see Howard and O'Garra, 1992; Moore et al., 1993). In addition, IL-10 augments proliferation and differentiation of B cells. It is mainly produced by the Th2 subset of T-cells (Fiorentino et al., 1989) but can also be produced by Ly-1 B cells, macrophages, thymocytes, keratinocytes, and activated mast cell lines (Moore et al., 1993). Murine IL-10 is a polypeptide existing as a mixture of 17, 19, and 21 kDa species (Moore et al., 1993) and is expressed in a wide range of tissues (Broski et al., 1994). TheIl10 gene localizes to mouse Chromosome 1 (Kim et al., 1992) at a position of 69.9 cM (Mouse Genome Database, 1997).
Development
Mice carrying a null-mutation of the Il10 gene (Il10tm1Cgn) were generated by Dr. Ralf Kuhn at the Institute of Genetics, University of Cologne (Kuhn et al., 1993). TheIl10 gene was disrupted in 129/Ola-derived embryonic stem (ES) cells by replacing codons 5-55 of the first exon by a linker, providing a termination codon and a neo gene, and by introducing a termination codon into exon 3. Transfected ES cells were injected into C57BL/6 blastocysts.
Description
Mice homozygous for the Il10 gene mutation do not produce IL-10 and completely lack IL-10 activity (Kuhn et al., 1993). Homozygous mutants on the mixed 129/Ola x C57BL/6J genetic background are growth-retarded by 3-4 weeks of age. Anemia results in 90% of mutants by 7-11 weeks, and mortality is about 30% prior to 3 months of age.
Il10tm1Cgn mutant mice spontaneously develop a chronic inflammatory bowel disease (IBD) (Kuhn et al., 1993; Lohler et al., 1995; Berg et al., 1996), with an incidence of 100% by 3 months of age (Berg et al., 1996). Clinical signs of inflammation are diarrhea, perianal ulceration, intestinal bleeding and occasional rectal prolapse (Kuhn et al., 1993; Lohler et al., 1995; Berg et al., 1996). Intestinal histopathology in Il10tm1Cgn mice shares some features with human Crohn's disease. The entire intestinal tract can be affected; lesions are segmental and variable (Kuhn et al., 1993; Lohler et al., 1995; Berg et al., 1996). The duodenum, proximal jejunum, cecum and proximal colon are most severely affected.
The inflammatory process is characterized by focal transmural inflammation, mucosal proliferation, focal ulceration and extensive infiltration with lymphocytes, plasma cells, macrophages, and scattered neutrophils. In addition, 60% of mutants develop colorectal adenocarcinoma by 6 months of age (Berg et al., 1996).
The viability and disease development of Il10tm1Cgn mutant mice is strongly dependent on their genetic background. Intestinal lesions are most severe in mutants on 129/SvEv and BALB/c backgrounds, of intermediate severity in 129/Ola x C57BL/6 outbred mutants, and least severe in C57BL/6J mutants (Berg et al., 1996). Environmental factors play an important role in the severity of inflammation as well. Il10tm1Cgn mutants kept under specific pathogen-free conditions exhibit only local inflammation confined to the proximal colon (Kuhn et al., 1993; Lohler et al., 1995).
The pathogenesis of the disease probably represents an enhanced T helper 1 (Th1) response to antigens of the enteric bacterial flora due to the lack of IL-10 that normally down-regulates such T cell reactivity. Mechanistic studies associate uncontrolled cytokine production by activated macrophages and CD4+ Th1-type T cells with IBD exhibited by Il10tm1Cgn mutant mice (Berg et al., 1996). Cell transfer experiments indicate that IBD in Il10tm1Cgn mutant mice is predominantly mediated by CD4+ Th1-type T cells (Davidson et al., 1996). The concept of a pathogenic Th1 response is further supported by showing that anti-interferon-y antibody treatment attenuates intestinal inflammation in young Il10tm1Cgn mutant mice (Berg et al., 1996). Consistent with this concept, Il10tm1Cgn mutant mice have an enhanced delayed type hypersensitivity reaction to antigens eliciting a strong Th1 response (Rennick et al., 1995). They are abnormally sensitive to bacterial lipopolysaccharide (Berg et al., 1995a), to infections with Toxoplasma gondii (Gazzinelli et al., 1996) and Plasmodium chabaudi chabaudi (Linke et al., 1996) and to skin irritants including those that elicit contact hypersensitivity reactions (Berg et al., 1995b).
Features of mice maintained at The Jackson Laboratory
The Jackson Laboratory maintains the Il10tm1Cgn mutation congenic on the C57BL/6J and C57BL/10J genetic backgrounds. The intestinal tracts of 9 C57BL/6J-Il10tm1Cgn mice and 6 C57BL/10J-Il10tm1Cgn mice, 5 weeks to 11 months of age, from barrier-maintained production colonies, were evaluated for histologic signs of IBD. Of the 15 mice evaluated, only 2 C57BL/6J-Il10tm1Cgn mice at the age of 9 months had any evidence of inflammation in the bowel. The inflammation observed was very mild, consisting of no more than two very small foci of crypt abscess formation (2-3 crypts) surrounded by a mixed inflammatory cell infiltrate or just lymphocytes in the distal colon. The overlying epithelium was eroded, suggesting that these mild lesions were traumatic rather than associated with the mutation.
A research colony of Il10tm1Cgn mutant mice on the mixed 129/Ola x C57BL/6J genetic background kept in a conventional mouse room consistently exhibited loose stools and rectal prolapse. Thirteen mice, ranging from 3 to 22 months of age, were examined histologically. All mice examined showed histologic signs of IBD. Lesions were distributed throughout the intestinal tract and included cystic hyperplasia of Brunner's glands in the duodenum, mononuclear cell infiltrates (consisting primarily of lymphocytes and plasma cells) into the lamina propria of villi in the small intestine, diffuse mononuclear cell infiltrates in the cecum and colon (Fig. 1), crypt abscesses (Fig. 1), crypt hyperplasia (Fig. 1-3), hyperplastic foci that perforated the muscularis mucosa and entered the submucosa (adenomatous hyperplasia) (Fig. 2, 3), ulcers of various sizes in the cecum and colon associated with acute inflammation (Fig. 4-6) and pseudopolyps adjacent to ulcers (Fig. 5). Lesions varied in severity among individuals and progressed with age.
These findings confirm reports about a genetic and/or environmental influence on disease expression inIl10tm1Cgn mutant mice.
Colony maintenance
The Il10tm1Cgn mutation is maintained under barrier conditions by The Jackson Laboratory Mouse Mutant Resource. Two congenic strains carrying the Il10tm1Cgn mutation are available: C57BL/10-Il10tm1Cgn (Stock# JR2250) and C57BL/6-Il10tm1Cgn (Stock# JR2251). Both strains are maintained by mating homozygous siblings. They are currently at N7F7 (Stock# JR2250) and N10F6 (Stock# JR2251). Only homozygous mice may be ordered. Il10tm1Cgn mutant strains should be maintained under pathogen-free conditions.
Genetic typing
A three primer PCR protocol may be used to distinguish homozygous, heterozygous, and normal wildtype mice. The PCR protocol is available athttp://www.jax.org; or through an e-mail inquiry to [email protected], or by calling 1-800-422-MICE.
Inquiries
For inquiries or to place a request for mice, contact the Customer Service Department either by telephone at 1-800-422-MICE or 207-288-5845, or by fax at 207-288-6150.
Figures
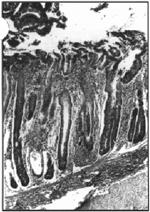 Figure 1. Colon with hyperplastic crypts separated by a mononuclear cell infiltrate into the lamina propria, crypt abscesses (arrow) and mononuclear cell infiltration into the submucosa. H&E, 125x.
Figure 1. Colon with hyperplastic crypts separated by a mononuclear cell infiltrate into the lamina propria, crypt abscesses (arrow) and mononuclear cell infiltration into the submucosa. H&E, 125x.
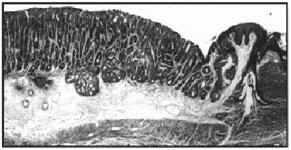 Figure 2. Marked mucosal hyperplasia of the terminal colon with invasion into the submucosa. H&E, 50x.
Figure 2. Marked mucosal hyperplasia of the terminal colon with invasion into the submucosa. H&E, 50x.
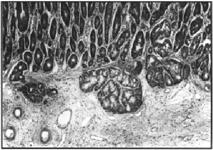 Figure 3. Higher magnification of figure 2. H&E, 125x.
Figure 3. Higher magnification of figure 2. H&E, 125x.
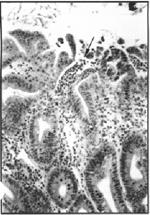 Figure 4. Small ulcer with neutrophils (arrow) streaming into the lumen of the cecum. H&E, 313x.
Figure 4. Small ulcer with neutrophils (arrow) streaming into the lumen of the cecum. H&E, 313x.
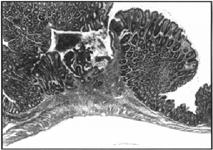 Figure 5. Colonic ulcer surrounded by pseudopolyps. H&E, 50x.
Figure 5. Colonic ulcer surrounded by pseudopolyps. H&E, 50x.
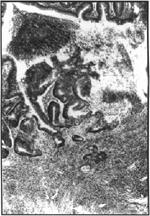 Figure 6. Higher magnification of figure 5. H&E, 125x.
Figure 6. Higher magnification of figure 5. H&E, 125x.
References
1. Berg DJ, Kuhn R, Rajewsky K, Muller W, Menon S, Davidson N, Grunig G, Rennick D. 1995a. Interleukin-10 is a central regulator of the response to LPS in murine models of endotoxin shock and the Shwartzman reaction but not endotoxin tolerance.J Clin Invest 96: 2339-2347.
2. Berg DJ, Leach MW, Kuhn R, Rajewsky K, Muller W, Davidson NJ, Rennick D. 1995b. Interleukin 10 but not interleukin 4 is a natural suppressant of cutaneous inflammatory responses.J Exp Med 182:99-108.
3. Berg DJ, Davidson N, Kuhn R, Muller W, Menon S, Holland G, Thompson-Snipes L, Leach MW, Rennick D. 1996. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4+ TH1-like responses. J Clin Invest 98: 1010-1020.
4. Broski AP, Halloran PF. 1994. Tissue distribution of IL10 mRNA in normal mice. Evidence that a component of IL10 expression is T and B cellindependent and increased by irradiation.Transplantation 57:582592.
5. Davidson NJ, Leach MW, Fort MM, Thompson-Snipes L, Kuhn R, Muller W, Berg DJ, Rennick DM. 1996. T helper cell 1-type CD4+ T cells, but not B cells, mediate colitis in interleukin 10-deficient mice. J Exp Med 184:241-251.
6. Fiorentino DF, Bond MW, Mosmann TR. 1989. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones.J Exp Med 170:2081 2095.
7. Gazzinelli RT, Wysocka M, Hieny S, Scharton-Kersten T, Cheever A., Kuhn R, Muller W, Trinchieri G, Sher A. 1996. In the absence of endogenous IL-10, mice acutely infected withToxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-(, and TNF-". J Immunol 157:798-805.
8. Howard M, O'Garra A. 1992. Biological properties of interleukin 10.Immunol Today 13: 198-200.
9. Kim JM, Brannan CI, Copeland NG, Jenkins NA, Khan TA, Moore KW. 1992. Structure of the mouse IL-10 gene and chromosomal localization of the mouse and human genes.J Immunol 148:3618-3623.
10. Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. 1993. Interleukin-10-deficient mice develop chronic enterocolitis.Cell 75:263-274.
11. Linke A, Kuhn R, Muller W, Honarvar N, Li C, Langhorne J. 1996. Plasmodium chabaudi chabaudi: differential susceptibility of gene-targeted mice deficient in IL-10 to an erythro- cytic-stage infection.Exp Parasitol 84:253-263.
12. Lohler J, Kuhn R, Rennick D, Rajewsky K, Muller W. 1995. Interleukin-10-deficient mice: a model of chronic mucosal inflammation, p. 401-407.In Tytgat GNJ, Bartelsman JFWM, Deventer SJH (ed.), Inflammatory bowel diseases. Kluwer Academic Publishers, Dordrecht.
13. Moore KW, O'Garra A, de Waal Malefyt R, Vieira P, Mosmann TR. Interleukin-10. 1993.Ann Rev Immunol 11:165-190.
14. Mouse Genome Database (MGD), Mouse Genome Informatics, The Jackson Laboratory, Bar Harbor, Maine. World Wide Web (URL: http://www.informatics.jax.org/). (June, 1997).
15. Rennick D, Davidson N, Berg D. 1995. Interleukin-10 gene knockout mice: a model of chronic inflammation.Clin Immunol Immunopathol 76:S174-S178.
