Uncovering the basis of type 1 diabetes using immunodeficient mouse models
There are multiple immune cells implicated in the development of T1D. Here we point to seven immunodeficient mouse models that helped researchers figure out which cell types mediate insulin-dependent diabetes mellitus and continue to spark novel intervention therapies.
1. Role of B and T cells
In fact, when NOD mice are deficient in B and T cells, they do not develop diabetes. That is the case with NOD.CB17-Prkdcscid/J (NOD scid) mice. On the other hand, if you transfer CD4+ and CD8+ T-cells from diabetic NOD mice into NOD scids - yes, you guessed - mice become diabetic. This was shown by JAX scientistsChristianson SW, Shultz LD, Leiter EH over 10 years ago in Christianson et al 1993.
a) NOD mice homozygous for the Cd8atm1Mak targeted mutation are deficient in functional cytotoxic T-cells; but they have normal helper T-cell development and function. With no cytotoxic T-cells these mice do not develop diabetes.
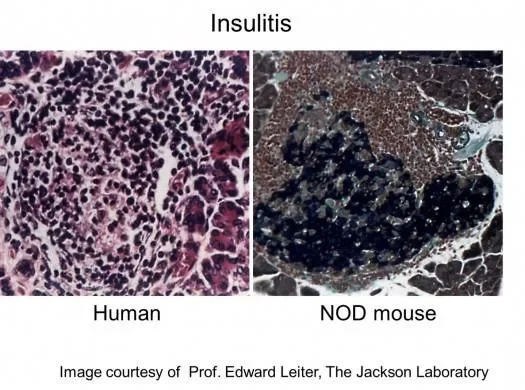
b) B cell KO NOD mice NOD.129S2-Ighmtm1Cgn/Dvs homozygous for the Ighm allele lack mature B-cells, are free of insulitis, and do not develop insulin-dependent diabetes.
2. Role of the innate immune system
The role of innate cells in the initiation of this disease is not well understood. Using an inbred non-obese diabetic (NOD) model, and a spectacular experimental design, Diana et al 2013 cleverly showed that diabetes onset involves crosstalk among the innate-like B-1a cells, neutrophils and plasmacytoid dendritic cells.

3. Role of HLA class I
Human patients express specific HLAs such as HLA-A2.1 (class I) are associated with increased T1D susceptibility. Mice expressing human class I MHC HLA-A2.1 molecules mediate beta cell auto-reactive CD8 T cell responses accelerating the rate of diabetes onset.
β2 microglobulin is necessary for cell surface expression of MHC class I. In the absence of MHC class I, CD8 T cells cannot develop. Several NOD strains deficient in MHC class I, are in fact free of diabetes. B2m KO mice are immunodeficient (with few CD8+ cytotoxic T-cells, NK cell deficiency, NK1+ T cells deficiency and decreased levels of serum Ig) and block both insulitis and autoimmune diabetes.
4. Experimental treatments using NSG mice
Researchers are experimenting with islet cell transplantation, which provides new insulin-producing cells from a donor pancreas. NOD-scid mice are a great platform for adoptive transfer of syngeneic mouse diabetogenic cells, but do not support human cells.
The NSG mouse model continues to be instrumental in the adoptive transfer model of human cells and has overcome serious technical barriers due totissue rejections of HLA-mismatched pancreatic islet grafts. New techniques and better drugs to prevent islet cell rejection may improve its future chance for success.
It is very exciting to see how research is transitioning from understanding the role of specific cell types that mediate T1D, to developing sustainable treatments that will eliminate the need for a lifelong commitment to taking insulin.
