NONcNZO10 LtJ a new JAX Mice model for obesity-induced type 2 diabetes
June 1, 2005
JAX® Mice strain NONcNZO10/LtJ (Stock Number 004456) is a recombinant congenic strain developed at The Jackson Laboratory specifically to model the more common form (so-called "garden variety") of human type 2 diabetes. Although JAX® Mice strains BKS.Cg-m +/+Leprdb/J (Stock Number 000642), B6.V-Lepob/J (Stock Number 000632), and KK.Cg-Ay/J (Stock Number 002468) are excellent models of monogenic obesity and useful for researching type 2 diabetes, they do not reflect the more common human obesity-induced diabetes (diabesity) syndromes.
Common human diabesity syndromes are polygenic, not monogenic, and the clinical phenotypes of the monogenic models are extreme: massive obesity and hyperphagia, either extremely high or no leptin in circulation, and extreme hyperinsulinism. In contrast, NONcNZO10/LtJ has moderate behavioral and endocrine phenotypes, and males exhibit a maturity-onset transition from impaired glucose tolerance to a stable non-fasting hyperglycemia.1,2 To develop hyperglycemia, this model must attain specified body weight thresholds during peripuberty and afterwards.
NONcNZO10/LtJ was constructed with two unrelated JAX® Mice strains, NON/LtJ (NON, Stock Number 002423) and NZO/HILtJ (NZO, Stock Number 002105), each with separate sets of quantitative trait loci (QTL) contributing to impaired glucose tolerance. NON males have elevated fasting blood glucose levels, impaired glucose tolerance, and low glucose-stimulated insulin secretion from isolated islets (indicating a defect in the pancreatic beta cells).3 NZO is a model of morbid and highly polygenic obesity associated with hyperphagia, marked hyperinsulinemia, and hyperleptinemia. Approximately 50% of NZO males, specifically those that gain weight most rapidly during peripuberty, develop maturity-onset diabesity.4,5 In contrast, NON males do not spontaneously transit from impaired glucose tolerance to overt diabetes. However, the NON genome contributes QTL that interact negatively with NZO-derived QTL to exacerbate diabesity in (NZO x NON)F1 males.5
Knowing the chromosomal locations of some of these diabesity QTL from both parental strains6 permitted development of NONcNZO10 by two cycles of backcrossing the NZO QTL into the NON genetic background followed by inbreeding with selection for the diabesity-promoting QTL.1,2 The diabesity phenotype conferred by this QTL combination very well models the more common human diabesity syndromes: moderate obesity without hyperphagia and reproductive failure, and only moderately elevated insulin and leptin concentrations in plasma.2
Modified NONcNZO10/LtJ husbandry yields more consistent phenotype
The NONcNZO10/LtJ strain has been under development in JAX® Services since November 2004. Originally, we distributed males when they were about eight weeks old and had been weaned onto an NIH-31 diet containing 6% fat (LabDiet® 5K52). All previously published phenotypes and histopathology of the diabesity syndrome spontaneously developed by NONcNZO10 males are based on this 6% diet.1,2,7,8 However, some customers reported that NONcNZO10/LtJ males weaned onto this diet did not become hyperglycemic (chronically elevated non-fasting glucose level of ≥250 mg/dl plasma) by the time they were 12 to 20 weeks old. So that males would exceed the weight thresholds necessary to force penetrance of hyperglycemia, we weaned them onto an NIH-31 diet containing 10-11% fat (LabDiet® 5K20). These males weighed more than those fed the 6% fat diet at each sampling point, weighing an average 6.5 grams more at the 20 week sampling point (Fig. 1). Over 90% of them were hyperglycemic by the time they were eight weeks old (Fig. 2). Histopathology of pancreas, livers, and kidneys from 22-week old males fed the 10-11% diet was similar to that reported for diabetic 24-week old males fed the 6% diet.2
Upon customer request, we will still distribute NONcNZO10/LtJ males weaned onto a 6% diet, but based on our experiences, we cannot guarantee how many of them will become hyperglycemic. Our standard procedure is to distribute males weaned onto a 10-11% fat diet. Customers wanting these mice to develop hyperglycemia in their facilities should continue to feed them the 10-11% diet.
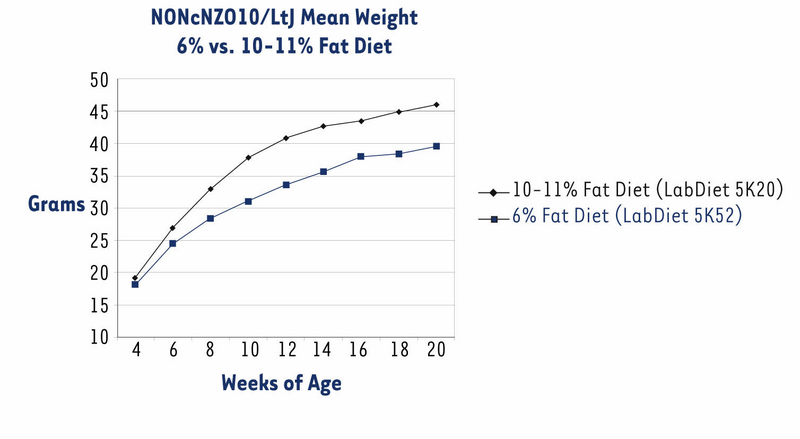
Figure 1. NONcNZO10/LtJ males fed a 10-11% fat diet weighed significantly more than those fed a 6% diet (P < 0.0025 for six-week olds; P < 0.0001 for eight-week olds and older; ANOVA with Least Squares Means contrast). Data show mean -/+ SEM for a group of 18-20 males fed the 6% diet and a group of 26 to 29 males fed the 10-11% diet.
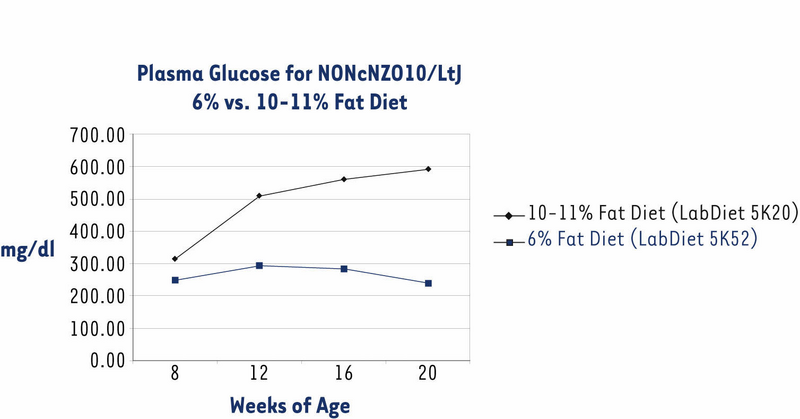
Figure 2. Non-fasting plasma glucose concentrations levels of NONcNZO10/LtJ males fed a 10-11% fat diet were significantly higher than those fed a 6% diet when both groups were over eight weeks old (P < 0.0001; ANOVA with Least Squares Means contrast). Data show mean -/+ SEM for the same group of males shown in Fig. 1.
Recommended controls
The controls recommended for NONcNZO10/LtJ depend upon the model’s intended use. If males are to be used for drug testing, vehicle-injected NONcNZO10/LtJ males may suffice. Otherwise, NON males are recommended as a genetic background control. NON and NONcNZO10/LtJ are about 88% identical genetically.1,2 As noted above, although NON males exhibit moderate adiposity and impaired glucose tolerance associated with poor insulin secretory responses to glucose, they develop neither the hyperglycemia nor the hypertriglyceridemia observed in NONcNZO10/LtJ males.
Because NONcNZO10/LtJ females weaned onto a 6% fat diet are normoglycemic, they can be compared to age-matched hyperglycemic males to evaluate the effects of chronic diabetes on specific phenotypes. However, NONcNZO10/LtJ females have yet to be tested for hyperglycemia when weaned onto a 10-11% fat diet. Initial results indicate that they are able to bring early rises in plasma glucose back into the normoglycemic range as they age.
NONcNZO5/LtJ (Stock Number 004455) males are excellent controls for comparative gene expression studies designed to identify new drug targets: NONcNZO5/LtJ contains diabesity-protective QTL at chromosomal positions where NONcNZO10/LtJ contains diabesity-susceptible QTL, and each have comparable body weights and visceral adiposity.2 Whereas glucose tolerance of NONcNZO5/LtJ males (like that of the parental NON males) is impaired, that of NONcNZO5/LtJ females is normal. In our studies, no NONcNZO5/LtJ males (0/31) fed a 6% fat diet became hyperglycemic; slightly over 10% (1/9) fed a 10-11% fat diet developed chronic hyperglycemia, but not until they were 20 weeks old.9 Because the 10-11% fat diet may combine with impaired glucose tolerance to disrupt glucose homeostasis, a higher frequency of males may develop chronic diabesity when fed the 20% fat diets typically used in diet-induced obesity studies.
In some cases, NZO, one of the parental strains used to develop NONcNZO10/LtJ, may be a good control. Penetrance of diabesity in males is variable, usually not exceeding 50%. Although they have peripheral leptin resistance, NZO mice have central leptin sensitivity, suggesting a defect in leptin transport across the blood-brain barrier.4,5 Males develop hypertension on a diet containing 20% fat.10
NZL/LtJ (Stock Number 005067), a recombinant congenic variant of NZO now available from The Jackson Laboratory, may be a good control for some studies. Although it has the same diabesity features as does NZO, it reproduces better than does NZO.11
Because most of the genetic background of NONcNZO10/LtJ is derived from NON, a Swiss-derived inbred strain, other Swiss-derived inbred JAX® Mice may be considered for metabolic controls. For example, SWR/J (Stock Number000689) is resistant to diet-induced obesity12 and does not exhibit impaired glucose tolerance,13 as do NON males and the closely-related ALS/LtJ strain (Stock Number 003072). Indeed, the ALS/Lt strain may be of interest to investigators because males are excellent models for an insulin-resistant metabolic syndrome that responds to treatment with anti-oxidants.14
We have a considerable number of new models of diabesity and metabolic syndrome. For more information, contact[email protected].
References:
(Authors in bold are Jackson Laboratory scientists)
1Reifsnyder PC, Leiter EH. 2002. Deconstructing and reconstructing obesity-induced diabetes (diabesity) in mice.Diabetes 51:825-32.
2Leiter EH, Reifsnyder PC. 2004. Differential levels of diabetogenic stress in two new mouse models of obesity and type 2 diabetes.Diabetes 53 Suppl 1:S4-11.
3 Sima AAF, Shafrir E 2000. Animal Models in Diabetes: A Primer. Taylor and Francis, Publ Amsterdam, Netherlands.
4 Andrikopoulos S, Thorburn AW, Proietto J. 2001. The New Zealand obese mouse: a polygenic model of type 2 diabetes. In Animal Models of Diabetes: A Primer. Sima AAF, Shafrir E, Eds. Amsterdam, Harwood Academic. pp. 171-84.
5Leiter EH, Herberg L. 1997. The polygenetics of diabesity in mice. Diabetes Revs 5:131-48.
6Leiter EH, Reifsnyder PC, Flurkey K, Partke HJ, Junger E, Herberg L. 1998. NIDDM genes in mice: deleterious synergism by both parental genomes contributes to diabetogenic thresholds. Diabetes 47:1287-95.
7 JAX Notes™ supplement # 12. 2004. The Jackson Laboratory. Data Sheet for JAX® Mice Strain NONcNZO10/LtJ.
8 The Jackson Laboratory. 2004. Type 2 Diabetes and Obesity Resource Manual.
9Xiao Q, Ji S, Miller L, Reifsnyder PC, Leiter EH, Mistry J. 2004. Characterization of new obesity/diabesity mouse models with a newly developed mouse adipokine multiplex assay using the luminex xMAP technology.Diabetes 53:A99.
10 Ortlepp JR, Kluge R, Giesen K, Plum L, Radke P, Hanrath P, Joost HG. 2000. A metabolic syndrome of hypertension, hyperinsulinaemia and hypercholesterolaemia in the New Zealand obese mouse. Eur J Clin Invest 30:195-202.
11 McInerney MF, Najjar SM, Brickley D, Lutzke M, Abou-Rjaily GA, Reifsnyder PC, Haskell BD, Flurkey K, Zhang YJ, Pietropaolo SL, Pietropaolo M, Byers JP, Leiter EH. 2004. Anti-insulin receptor autoantibodies are detected in type 2 diabetes-prone NZO-Lt mice using a new flow cytometric assay.Exptl Diabesity Res 5:177-86.
12 West DB, Boozer CN, Moody DL, Atkinson RL. 1992. Dietary obesity in nine inbred mouse strains. Am J Physiol 262:R1025-32.
13Leiter EH, Coleman DL, Hummel KP. 1981. The influence of genetic background on the expression of mutations at the diabetes locus in the mouse. III. Effect ofH-2 haplotype and sex. Diabetes 30:1029-34.
14 Mathews CE, Bagley R, Leiter EH. 2004. ALS/Lt: a new type 2 diabetes mouse model associated with low free radical scavenging potential.Diabetes 53 Suppl 1:S125-9.
