Microphthalmia and ocular infections in inbred C57 black mice
JAX Notes | October 2, 1995Several strains of inbred black mice, originally derived by W.L. Russell of The Jackson Laboratory in 1921, were designated as C57 Black (C57BL) after approximately the thirty-second generation of inbreeding. Russell's lines 6 and 10 were further developed by Chase, who found abnormal eyes in both strains 4.3% of the time (Chase, 1941; Chase, 1942). Asymmetric microphthalmia and "anophthalmia" were found in affected mice.
Microphthalmia and anophthalmia are 6.2 times as common in females and 5.8 times as common in the right eye. Congenic strains of C57BL/10 mice [B10.A, B10.BR, B10.D2, B10.A(2R), B10.A(5R), B110.A(15R), B10.A(1R), B10.A(18R), and B10.OL] have an incidence of clinical microphthalmia ranging from 0.8 to 9.2% (Pierro and Spiggle, 1967). While the individual strains of C57BL mice are genetically identical within the strain, they all show the same propensity to develop sporadic microphthalmia or anophthalmia. The tendency to manifest these abnormalities does not represent a specific mutation but rather the possibility of developing microphthalmia or anophthalmia, perhaps due to "environmental" influences. This is seen in striking fashion in the response to fetal alcohol exposure (see below).
Gross lesions
Most of the published studies base the ocular diagnosis on gross examination alone. The reported incidence of ophthalmic abnormalities varies from 4.4% (Chase, 1942) to 10% (Kalter, 1968). True anophthalmia is unusual and can only be diagnosed by careful histopathologic examination (Smith, 1994). The relative incidence of microphthalmia versus anophthalmia has never been established since no large series has been examined microscopically.
Affected mice often develop recurrent ocular infections. These infections are the result of the small or absent eyes with consequent poor drainage of tears and debris, rather than being due to poor animal husbandry. The investigator must recognize that the described ophthalmic abnormalities represent a background feature of inbred black mice in order to avoid incorrect interpretation of experiments that involve the eyes in these mice. Figure 1 shows a C57BL/6J mouse with moderate microphthalmia.
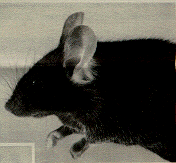
Figure 1. C57BL/6J mouse with moderate microphthalmia. The eye appears partly closed because the small eye does not support the lids.
The presence of corneal and opacities and anterior polar cataracts in inbred C57 black mice has been known for many years (Koch and Gowen, 1939) and is easily identified with slit lamp biomicroscopy. The incidence of both cataracts and corneal opacities is quite variable. Corneal opacity (Figure 2) may be associated with a keratolenticular adhesion. The corneal opacity is due to persistence of the epithelial stalk of the lens vesicle, which normally disappears around day 11 of gestation (Koch and Gowen, 1939; Rugh, 1968). The corneal opacity is seen both with and without microphthalmia.
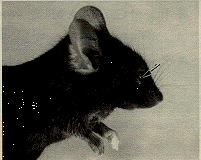
Figure 2. C57BL/6J mouse with corneal opacity (arrow), due to persistence of the stalk of the lens vesicle.
Microscopic lesions
One of both eyes may be affected. The ocular changes may range from a cataract and minor changes in the anterior segment to complete anophthalmia. In mice with corneal involvement, the corneal opacity is located centrally and is less than one millimeter in diameter. The lens is attached to the posterior corneal surface, and there is absence of the central anterior lens capsule. Lens epithelium passes through this area and becomes continuous with the surface corneal epithelium (Figure 3).

Figure 3. Eye lesions in C57BL/6J mouse. The lens (L) is adherent to the cornea. There is a defect in the corneal stroma (S) that is filled with epithelial cells. The corneal epithelium (arrowheads) is continuous with the lens epithelium (arrows). H & E staining magnified x 200.
Cataracts
The cataracts found in inbred C57 black mice with microphthalmia may be characterized by minor cortical degeneration, by major involvement of the cortex and nucleus, or by extrusion of lens cortex through a dehiscence in the lens capsule. The lens cortical material in an extrusion cataract may be deposited throughout the eye (Figure 4).
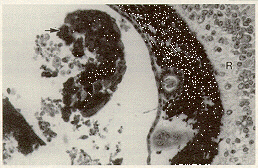
Figure 4. C57BL/6J mouse with severe microphthalmia. Lens cortical material lies free in the vitreous (arrow). A layer of abnormal pigment covers the surface of the retina (R). H & E staining magnified x 100.
The iris and ciliary body may be poorly differentiated and the anterior chamber absent (Figure 5).

Figure 5.C57BL/10J mouse with moderate microphthalmia. The lens (L) demonstrates a cataract. The anterior portion of the eye is poorly differentiated and consists of a mass of pigmented cells (arrows). The retina contains a single dysplastic rosette (arrowhead). H & E staining magnified x 50.
The retina of affected mice may be undifferentiated and demonstrate folding, detachment or form dysplastic rosettes. With such severe involvement, the eye is often very small and detectable only by microscopic examination.
Anophthalmia
True anophthalmia does occur in inbred C57 black mice. The empty orbit of this mouse consists of a cavity lined by conjunctiva, separated anteriorly by the lids, and posteriorly by the Harderian gland (Figure 6).
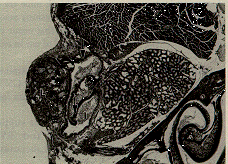
Figure 6. C57BL/6J mouse with anophthalmia. The lids (L) are fused, and the empty eye socket is lined by conjunctival epithelium (arrows). An inflammatory infiltrate (I) partly fills the empty socket. H & E staining magnified x 50.
Because the normal flushing action of the tears cannot occur, ocular secretions and debris may accumulate in the empty orbit and encourage development of secondary infections by opportunistic organisms. Isolates obtained in our laboratory includePasteurella pneumotropica, Corynebacterium spp., Lactobacillus spp., and coagulase negative Staphylococcus spp. (Sundberg, et al. 1990, 1991, Smith, et al., 1994).
Environmental effects
C57BL mice have been used to develop a mouse model of fetal alcohol syndrome (Sulik, et. al., 1981). Pregnant mice given intraperitoneal injections of 25% ethanol around the seventh day of pregnancy developed elevated blood ethanol levels. The rate of ocular malformations including microphthalmia and anophthalmia, (43.5% of the exposed mice) was four times that of the control C57BL mice (Sulik, et. al., 1981). An increased incidence of corneal opacities was also demonstrated. All defects were less common in other strains, after alcohol exposure. Alcohol appears to enhance the inherent susceptibility of inbred black mice to develop ocular malformations (Webster, et. al., 1983, Cook, et. al., 1987).
Embryology
Interaction between the forming lens at 9-10 days of embryonic life and the edges of the optic cup appears critical for proper formation of the eye (Coulombre and Coulombre, 1977; Harch, et. al., 1978). Size and location of the lens relative to the optic cup exerts an influence on formation of nearly all other structures of the mature eye. This complex subject is reviewed in detail elsewhere (Smith, et. al., 1994).
Analogous human disease
The "fetal alcohol syndrome" is a consequence of consumption of alcohol during pregnancy. Affected children demonstrate postnatal growth deficiency, central nervous system involvement with intellectual impairment, and a typical facial appearance that may include microphthalmia, central corneal opacities, microcephaly and maxillary flattening (Chen, et. al., 1991).
A group of spontaneous, occasionally familial, anterior chamber, iris, cornea and lens malformations that share some findings in common are referred to as anterior segment dysgenesis. There may be central absence of Descemet's membrane, irido-corneal adhesions, corneal-lenticular adhesions, cataract, enlargement of Schwalbe's line, corneal opacities and anterior synechias (Waring, et. al. 1975). These changes resemble those seen in the anterior segment of inbred black mice (Smith, et. al., 1994). The different forms of anterior segment dysgenesis have been associated with a variety of chromosomal abnormalities involving human chromosomes 4, 9, 11, 13-15, 18, 21, and X (reviewed in Smith, 1994), with autosomal dominant and recessive inheritance patterns (Green and Johnson, 1986).
Analogous animal disease
Microphthalmia and anophthalmia are also seen in mutations at specific loci (Chase, 1941), including fidget (fi) (Gruneberg, 1943); the microphthalmia (mi) locus (Grobman, 1947); Pax6Sey (Clayton and Campbell, 1968); and vacuolated lens with microphthalmia (Cat3vl) (Kratochvilova, 1981). Other laboratory animals reported with anophthalmia or microphthalmia include rats (Rao and Sesikeran, 1992), guinea pigs (Komich, 1971), dogs (Zhang, et. al., 1991) and pigs (Szabo, 1989).
Common uses of inbred C57 black mice
Inbred black mice are used for ophthalmic studies, particularly dealing with the fetal alcohol syndrome. Since lesions associated with fetal alcohol syndrome are identical, but increased in frequency, to those that occur spontaneously, results should be interpreted with caution. Inbred black mice may also be of use in evaluation of lens embryogenesis.
Availability
Many different strains of inbred C57 black mice are available at The Jackson Laboratory, including, C57BLKs/J, C57BL/6ByJ, C57BL/6J, C57BL/10J, C57BL/10SnJ as well as numerous congenic and recombinant inbred strains.
This work was supported in part by grants from The Sight Conservation Society of Northeastern New York (RSS), CA34196 from the National Cancer Institute (RSS, JPS), and funds from The Jackson Laboratory (JPS, RSS).
The authors wish to thank D. Boggess and J. Mill for technical assistance.
References
Chase HB. Studies on an anophthalmic strain of mice: III. Results of crosses with other strains. Genetics 27:33-48, 1942.
Chase HB, Chase EB. Studies on an anophthalmic strain of mice: I. Embryology of the eye region. J. Morphol 68:279-301. 1941.
Kalter H. Sporadic Congenital Malformations of Newborn Inbred Mice. Teratology 1:193-200, 1968.
Smith, RS, Roderick TH, Sundberg JP. Microphthalmia and Associated Abnormalities in Inbred Black Mice. Lab Animal Med 44:551-60, 1994.
Gruneberg H. Two new mutant genes in the house mouse. Genet J. 45:22-28, 1943.
Clayton RM, Campbell JC. Small eye, a mutant in the house mouse apparently affecting the synthesis of extracellular membranes. J. Hered. 38:381-384, 1968.
Kratochvilova J. Dominant cataract mutations detected in offspring of gamma-radiated male mice. J. Hered. 72:234-237, 1981.
Rao SH, Sesikeran B. Congenital anophthalmia in CFY rats; a newly identified autosomal recessive mutation. Lab Animal Sci. 42:623-625, 1992.
Komich RJ. Anophthalmos: an inherited trait in a new stock of guinea pigs. Am J Vet Res. 32:2099-2105, 1971.
Coulembre AJ, Coulombre JL. Abnormal organogenesis in the eyes, P. 329-341. In JG Wilson and F.C. Fraser (ed.), Handbook of Teratology, Vol. 2, Plenum Press, New York, NY. 1977.
Zhang RL, Samuelson DA, Zhang ZG, Reddy VN, Shastry BS. Analysis of eye lens-specific genes in congenital hereditary cataracts and microphthalmia of the miniature schnauzer dog. Invest Ophthalmol Vis Sci. 32:2662-2665, 1991.
Szabo K. Congenital malformations of various organ systems, p. 245-268. In Congenital malformations in laboratory and farm animals. Academic Press, San Diego, CA. 1989.
Kock FLP, Gowen JW. Spontaneous ophthalmic mutation in a laboratory mouse. Arch Pathol Lab Med. 28:171-176, 1939.
Pierro LJ, Spiggle J. Congenital eye defects in the mouse: I. Corneal opacity in C57 black mice. J Exp Zool. 166:25-38, 1967.
Chan T, Bowell R, Keefe ME, Lanigan B. Ocular manifestations in fetal alcohol syndrome. Brit J Ophthalmol. 75:524-526, 1991.
Sulik KK, Johnston MC, Webb MA. Fetal alcohol syndrome: embryogenesis in a mouse model. Science 214:936-938, 1981.
Webster WS, Walsh SE, McEwen SE, Lipson AH. Some teratogenic properties of ethanol and acetaldehyde in C57BL/6J mice: implications for the study of the fetal alcohol syndrome. Teratology 27:331-343, 1983.
Cook CS, AZ Nowotney, andKK Sulik. Fetal alcohol syndrome: eye malformation in a mouse model. Arch Ophthalmol. 105:1576-1581, 1987.
Rugh R. The mouse: its reproduction and development, p. 45. Minneapolis: Burgess Publishing Co., Minneapolis, MN. 1968.
Harch C, Chase HB, Golsalves NI. Studies on an anophthalmic strain of mice: VI. Lens and cup interaction. Devel Biol. 63:352-357, 1978.
Sundberg JP, Brown KS, Bedigian H. Ulcerative blepharitis and periorbital abscesses in BALB/cJ and BALB/cByJ mice. JAX Notes 443:3-4, 1990.
Sundberg JP, Brown KS, Bates R, Cunliffe-Beamer TL, Bedigian H. Suppurative conjunctivitis and ulcerative blepharitis in 129/J mice. Lab Anim Sci. 41:516-518, 1991.
Waring GO, Rodrigues MM, Laibson PR. Anterior chamber cleavage syndrome: a stepladder classification. Surv Ophthalmol. 20:3-27, 1975.
Green JS, Johnson GJ. Congenital cataract with microcornea and Peters' anomaly as expressions of one autosomal dominant gene. Ophthalmic Pediatr Genetics 7:187-194, 1986.
