Immuno-oncology – that is, pharmacologically stimulating a patient’s immune responses to destroy malignant tumor cells – has been a long hoped-for strategy for treating cancer. Compounds that act through the patient’s immune system have the potential to avoid off-target toxicities and unwanted side effects that often are associated with more traditional chemotherapeutic and radiation-based strategies.
Frustratingly, immuno-oncology-based cancer therapies are way behind conventional cancer treatment in Pharma pipelines. But as scientists have learned more about the interactions and strategies that malignant cells use to outwit the immune system to avoid destruction, immuno-oncology-based compounds, such as Merck’s KEYTRUDA® (pembrolizumab)and Bristol-Myers Squibb’s OPDIVO® (nivolumab) – both programmed death 1 (PD-1) inhibitors - are finally starting to hit the clinics.
Animal models with both human tumors and functional human immune cells
The development of further immuno-oncology-based drugs would undoubtedly benefit from the use of pre-clinical small animal models that support the engraftment of clinically-relevant human tumors to validate candidate human immune cell modulators. In order to do this, the models must be immunocompromised in order to prevent graft rejection—a large problem for the field. A number of immunodeficient mouse models have been successfully used in the past to model human tumors to varying degrees, however, many tumors are difficult to engraft in mice, and of those that do engraft, many do not accurately reflect the characteristics seen in the patient.
That’s where JAX’s NSG™ (005557) mice come into play. Not only do NSG™ mice support the engraftment of the widest array of patient-derived xenografts (PDX) compared to other immunodeficient models; they can also be “humanized” to express human immune cells. By co-engrafting mice with human tumors that retain the same characteristics and human immune cells, a revolutionary platform to study the complex relationship between the human immune system and tumors is now created. For immuno-oncology researchers, that means they can test the efficacy of their compound to mediate human immune cell responses to kill patient-derived tumors, in a model that more closely represents a human patient.
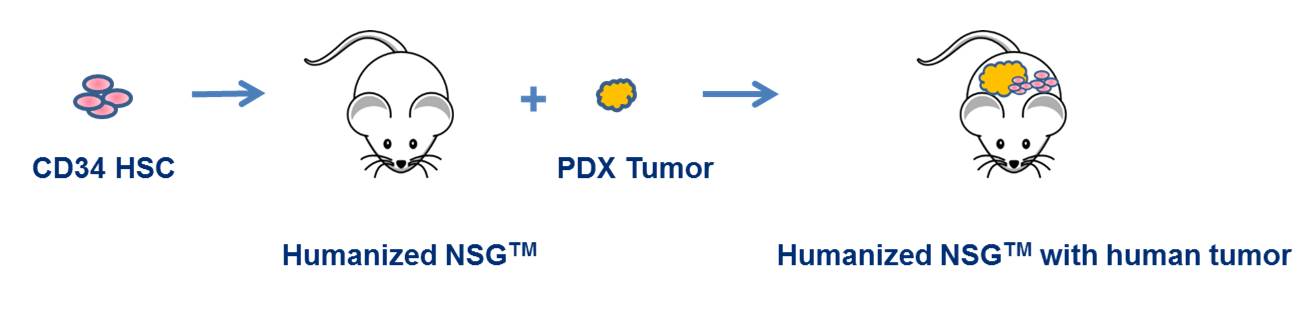
Figure 1. PDX-engrafted humanized-NSG™ models represent the next step in cancer modeling. They offer a unique platform for examining human T cell-dependent and B cell-dependent responses to clinically relevant tumors and for evaluating the efficacy of novel compounds to harness the immune system to attack specific tumor types.
PDX tumors engrafted into NSG™ mice retain a higher degree of their morphological and stromal cell characteristics, and show a lower degree of fibrosis and reduced infiltration of mouse-derived lymphocytes (see, for example, Simpson-Abelson MR et al. 2008).
JAX’s In Vivo Pharmacology Services group has begun to validate this remarkable model. Here’s a sneak peak at what they’ve found:
1. PDX tumors engraft and grow well in CD34+ hHSC (hu-CD34) humanized NSG™ mice.
Humanizing NSG™ does not impact PDX tumor growth rate. All of the tumors from our PDX cancer resource that we have tested so far- including those of different types and derived from different tissues - grow just as well or nearly so in hu-CD34 humanized NSG™ mice as in non-humanized hosts.
2. PDX tumor-engrafted hu-CD34 humanized NSG™ mice respond to standard of care treatments as expected.
So far, our data demonstrates that PDX tumors efficiently engraft in humanized NSG™ mice and respond to multiple SOC treatments. SOCs that we have tested include Avastin (bevacizumab), an angiogenesis inhibitor, fluorouracil (5-FU), a cell proliferation inhibitor, cisplatin, and docetaxel. All SOCs tested to date have inhibited growth of patient--derived tumors.
3. PDX tumor-engrafted hu-CD34 humanized NSG™ mice respond to an immune cell modulator.
With respect to immuno-oncology therapeutics, an important question is do the human immune cells that have differentiated from the hHSCs engrafted into the humanized NSG™ mice respond to anti-cancer immune cell modulators? The answer is overwhelmingly, “Yes”!
As shown in Figure 2, a triple negative breast PDX tumor (TM00098) engrafted into hu-CD34 humanized NSG™ mice show reduced tumor progression when treated with the anti-PD1 antibody and immune modulator KEYTRUDA®:
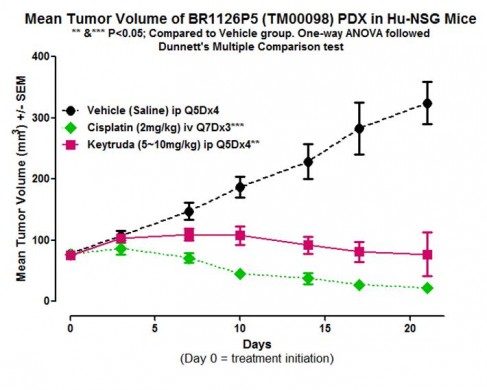
Figure 2. Keytruda® and cisplatin therapies show efficacy in PDX-engrafted, hu-CD34 NSG™ mice. The TNBC PDX tumor TM00098 was engrafted into hu-CD34 NSG™ mice and treated as indicated. Data from In Vivo Pharmacology Services, The Jackson Laboratory.
NSG™ provides a highly validated platform that supports great Patient-to-PDX tumor fidelity and, thus, a more accurate representation of the human tumor microenvironment. Our demonstration that PDX tumors grow efficiently in humanized mice and that these tumors respond favorably to both established SOC treatments and important new immune modulators suggests that humanized, PDX-engrafted NSG™ mice may be an affordable approach to validate single immune-based therapies as well as combination therapies pre-clinically without putting patients at risk.
Did you know that study-ready cohorts of Humanized NSG are readily available for shipment to your institution?
Stay tuned for future developments in this exciting field!