Decreased resilience of cardiac tissue over time might be due to changing macrophage types
Cardiovascular disease is a significant cause of death in many countries, and many of these cases are attributable to inadequate repair following cardiac injury. Tissue-associated macrophages play an integral role in the homeostasis, development and repair of the tissues in which they reside. In a recent study in the Journal of Experimental Medicine, Molawi et al. demonstrate that the constituent macrophages in heart tissue change over time from self-renewing, embryo-derived macrophages to less proliferative, monocyte-derived macrophages. This shift in the macrophage populations present in cardiac tissue may significantly impact heart tissue maintenance and repair, and identifies these macrophages as potential therapeutic targets for individuals with cardiovascular disease.
Four cardiac macrophages subtypes identified in heart
Using a stringent flow cytometry gating system, the authors measured the constituent macrophage types in postnatal B6.129P-Cx3cr1tm1Litt/J (005582) mice (CX3CR1GFP/+). These mice express GFP in cells that express Cx3cr1 (chemokine (C-X3-C motif) receptor 1), which include monocytes, dendritic cells, NK cells and macrophages. The authors characterized cardiac-specific macrophages as cells from heart tissue that express core macrophage signature markers Cd14, Cd64, MerTK, and F4/80. These cardiac macrophages could be further separated into MHC class II (MHCII)-positive or –negative populations. In analyses of heart cells prepared from newborn CX3CR1GFP/+ mice the majority of embryo-derived cardiac macrophages are Cx3cr1+MHCII-, but over time the cardiac macrophage population gradually diversifies into four different subtypes (Cx3cr1-MHCII-, Cx3cr1-MHCII+, Cx3cr1+MHCII-, and Cx3cr1+MHCII+) with the embryo-derived, Cx3cr1+MHCII- cells being in the minority by full adulthood (30 weeks). Fate mapping analysis using mice bred from Cx3cr1cre mice (B6J.B6N(Cg)-Cx3cr1tm1.1(cre)Jung/J, 025524) and a ROSA26-YFP reporter strain (B6.129X1-Gt(ROSA)26Sortm1(EYFP)Cos/J, 006148) to generate mice that have EYFP fluorescence in Cx3cr1-expressing cells (CX3CR1cre:R26-YFP), demonstrate that all adult cardiac macrophages originate from Cx3cr1+ cells, suggesting either that embryo-derived cardiac macrophages persist and differentiate or embryo-derived cardiac macrophages are replaced by adult monocyte-derived cells over time.
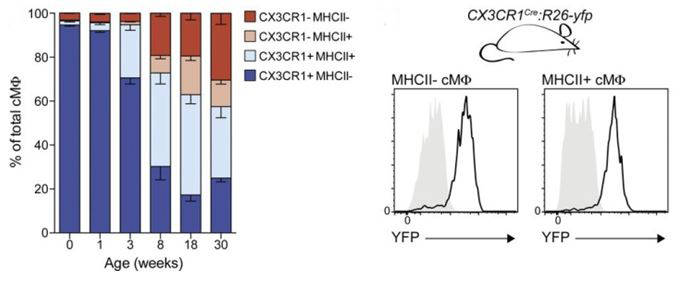
Cardiac macrophages develop into four subpopulations after birth. Mean percentage (Left) of cardiac macrophage subpopulations from CX3CR1GFP/+ mice of indicated age. Error bars represent SEM (n = 3–4). (Right) MHCII− and MHCII+ cardiac macrophages from adult CX3CR1cre:R26-yfp mice (black line) were analyzed for YFP expression and compared with Cre− littermate controls (gray area; n = 3–4). Data in all panels are representative of at least two independent experiments.
Monocyte-derived cardiac macrophages have decreased self-renewal capacity
To determine the origin of adult cardiac macrophages, Molawi et al. performed another fate mapping analysis using cardiac macrophages isolated from mice expressing a tamoxifen-inducible Cxcr1cre (B6.129P2(C)-Cx3cr1tm2.1(cre/ERT2)Jung/J, 020940) combined with the ROSA26-YFP reporter. In these mice, a pulse of tamoxifen at defined embryonic stages allows for YFP labeling of progenitor Cx3cr1+ cardiac macrophages, which can then be followed over time. The percentage of YFP+ in all cardiac macrophage types declines from birth to adulthood, supporting a mechanism in which embryonic cardiac macrophages are replaced with age by infiltrating monocyte-derived cells. The researchers also found that:
- Monocytes can recapitulate all major cardiac macrophage subtypes in BM chimera and parabiosis experiments
- Adult cardiac macrophages have reduced proliferation rates as measured by BrdU incorporation and Ki67 staining
These results demonstrate that the resident macrophage population in cardiac tissue changes dynamically with age and that the cardiac macrophages of older mice are unable to self-renew at the same high rate at which embryo-derived cardiac macrophages do. The difference in constituent populations suggests that cardiac macrophage functionality, also, is significantly changed over time and, thus, identifies these cell types as possible therapeutic targets for individuals with cardiovascular disease or recovering from ischemic injury.