Background
Helicobacter (H.) species (spp.) are Gram-negative, microaerophilic, spiral-to-curve-shaped bacteria with sheathed flagella. These bacteria are common colonizers of the gastrointestinal tract. Some species are associated with chronic gastrointestinal diseases in both humans and animals (Blaser, 1992; Fox and Lee, 1993). Recently, four species of Helicobacter, H. muridarum (Lee et al., 1992), "H. (Flexispira) rappini" (Schauer et al., 1993), H. hepaticus (Fox et al., 1994), and H. bilis (Fox et al., 1995) have been identified in gastrointestinal tracts of mice. These Helicobacter spp. primarily colonize the lower intestinal tract; H. hepaticus and H. bilis have also been found in the liver and bile of mice (Fox et al., 1994; Ward et al., 1994a; Ward et al., 1994b; Fox et al., 1995).
The pathogenic potential of the murine Helicobacter spp. is unclear. H. muridarum may occasionally colonize the stomach (Lee et al., 1992) and elicit gastritis (Queiroz et al., 1992). H. hepaticus and H. bilis are associated with chronic, active hepatitis (Ward et al., 1994a; Ward et al., 1994b; Fox et al., 1995); Koch's postulates have been fulfilled for the association of H. hepaticus with hepatitis (Ward et al., 1994a; Ward et al., 1994b; Fox et al. 1996b). H. hepaticus may also induce liver tumors (both adenomas and carcinomas) in older male A/JCr mice (Ward et al., 1994a; Ward et al., 1994b) and inflammatory large bowel disease in severe combined immunodeficiency (scid/scid) and nude (Hfh11nu/Hfh11nu) mice (Ward et al., 1996a).
Various mouse strains were reported to differ greatly in susceptibility to H. hepaticus-induced liver disease with A/JCr and scid/scid mice being highly susceptible and C57BL/6NCr mice being resistant (Ward et al., 1994a; Ward et al., 1994b). Male mice were more severely affected than females. The earliest histopathologic liver lesions seen, at 2 to 6 months of age, were focal necrosis and focal non-suppurative inflammation. By 6 to 10 months of age, extensive liver involvement included hepatocytomegaly, bile ductular (oval cell) hyperplasia, and cholangitis.
Little is known about the epidemiology of H. hepaticus infection and the pathogenesis of the associated hepatic disease. The organism is most likely transmitted by fecal-oral route (Ward et al., 1994a; Fox et al., 1996b). Motility due to the presence of flagella is probably important for colonizing the intestinal crypts (Fox et al., 1994). The mode of liver invasion is unknown. Production of urease and a soluble hepatotoxic factor may be involved in the pathogenesis of H. hepaticus-induced liver lesions (Fox et al., 1994; Taylor et al., 1995). A role for autoimmunity in progressive hepatitis and carcinogenesis in livers infected with H. hepaticus has further been suggested (Ward et al., 1996b).
H. hepaticus is susceptible to a number of antibiotics (Foltz et al., 1995; Russell et al., 1995; Foltz et al., 1996). Oral gavage and dietary administration of amoxicillin in combination with metronidazole and bismuth was effective in eradicating H. hepaticus from the intestinal tract and liver of mice (Foltz et al., 1995; Foltz et al., 1996).
Diagnosis
The presence of Helicobacter spp. in rodents is usually detected by means of polymerase chain reaction (PCR), bacteriologic culture, or histology. The testing program for Helicobacter spp. at The Jackson Laboratory currently includes these diagnostic methods.
PCR assays for the detection of murine Helicobacter spp. are based on the amplification of 16S rRNA gene sequences. These assays are conducted on DNA obtained from feces or tissues (large intestine, liver). Two H. hepaticus species-specific PCR assays (Battles et al., 1995; Shames et al., 1995) and a Helicobacter genus-specific PCR (Riley et al., 1996) have been developed. In the latter, restriction enzyme analyses of the amplified product are able to differentiate among the murine Helicobacter spp. but cannot differentiate H. bilis from "H. rappini". To distinguish H. bilis, an additional PCR assay needs to be performed.
 Murine Helicobacter spp. are isolated by microaerophilic culture of large intestine, liver or feces (Fox et al. 1994; Fox et al., 1995; Shames et al., 1995). Filtration (0.45-: pore size) of intestinal or fecal material increases the probability of a positive culture result for H. hepaticus by removing most other bacteria (Shames et al., 1995). Helicobacter speciation cannot be determined by colony morphology (thin spreading layer) and requires additional biochemical or electron microscopic characterization (Fox et al., 1995; Mahler et al., submitted).
Murine Helicobacter spp. are isolated by microaerophilic culture of large intestine, liver or feces (Fox et al. 1994; Fox et al., 1995; Shames et al., 1995). Filtration (0.45-: pore size) of intestinal or fecal material increases the probability of a positive culture result for H. hepaticus by removing most other bacteria (Shames et al., 1995). Helicobacter speciation cannot be determined by colony morphology (thin spreading layer) and requires additional biochemical or electron microscopic characterization (Fox et al., 1995; Mahler et al., submitted).
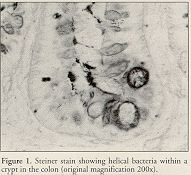
Histologic evaluation is done on large intestine or liver using Steiner's modification of the Warthin-Starry silver stain to visualize the helical bacteria (Ward et al., 1994b; Ward et al., 1996a) (Figure 1) . Histologic differentiation among the murine Helicobacter spp. is virtually impossible (Mahler et al., submitted).
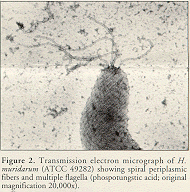 Electron microscopy can be used on cultural isolates or tissue samples. All known murine Helicobacter spp. except H. hepaticus have periplasmic fibers and multiple bipolar flagella (Figure 2).
Electron microscopy can be used on cultural isolates or tissue samples. All known murine Helicobacter spp. except H. hepaticus have periplasmic fibers and multiple bipolar flagella (Figure 2).
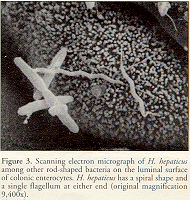 H. muridarum has two to three spiral turns (Lee et al., 1992), whereas "H. rappini" (Schauer et al., 1993) and H. bilis (Fox et al., 1995) appear fusiform to slightly curved. H. hepaticus is a curved-to-spiral rod with single bipolar flagella (Fox et al., 1994) (Figure 3).
H. muridarum has two to three spiral turns (Lee et al., 1992), whereas "H. rappini" (Schauer et al., 1993) and H. bilis (Fox et al., 1995) appear fusiform to slightly curved. H. hepaticus is a curved-to-spiral rod with single bipolar flagella (Fox et al., 1994) (Figure 3).
An enzyme-linked immunosorbent assay (ELISA) for anti-H. hepaticus antibodies has been described by Fox et al. (1996b). Elevated serum IgG H. hepaticus antibodies were only found in older A/JCr mice with H. hepaticus-induced liver damage but not in younger A/JCr mice with intestinal H. hepaticus colonization and without significant hepatitis. Even in germfree mice experimentally infected with H. hepaticus, the ELISA values were not appreciably elevated until the animals had been infected for almost a year (Fox et al., 1996a). The usefulness of serologic tests for diagnosis of Helicobacter spp. infections in mice remains to be determined.
Hysterectomy is an effective method for eliminating Helicobacter spp. from mice
In the 90’s, shortly after the initial detection of Helicobacter spp. in some strains of mice maintained at The Jackson Laboratory, a three-phase program was initiated to rebuild our colonies from Helicobacter-negative mice. The first phase involved rebuilding the standard inbred strains from mice that were rederived by hysterectomy into foundation stocks. In the second phase of our Helicobacter eradication program special strains and stocks were rebuilt.
A summary of test results for colonies descended from Helicobacter-negative mice (phase 1 of our program) is shown in the following table:
PCR Assay with primers detecting H. hepaticus, H.bilis, H. muridarum, and "H. rappini":
Foundation Stocks 0/319 (number of positive mice/number of mice tested)
Production Stocks 0/5164
Pedigreed Expansion Stocks 0/163a ; 0/303b
a Includes 25 specimens tested at the University of Missouri.
b Includes 176 mice submitted for culture to Microbiological Associates, Inc., and 5 mice submitted to the Massachusetts Institute of Technology.
In addition, we have found no association of Helicobacter spp. with liver lesions in mice at The Jackson Laboratory. Review of liver tumors from our archival collection of tissues failed to detect any evidence of Helicobacter spp. infections by both PCR and histology (Steiner's stain).
The health status of the mice that you receive is indicated on the Health Status Report that accompanies the mice. If you would like more information about the Helicobacter status or about the microbial profile of JAX mice feel free to contact the Laboratory Animal Health Office by telephone at 207-288-6205 or by fax at 207-288-6276.
References
1. Battles JK, Williamson JC, Pike KM, Gorelick PL, Ward JM, Gonda MA. 1995. Diagnostic assay for Helicobacter hepaticus based on nucleotide sequence of its 16S rRNA gene. J Clin Microbiol 33:1344-1347.
2. Blaser MJ. 1992. Helicobacter pylori: its role in disease. Clin Infect Dis 15:386- 391.
3. Foltz CJ, Fox JG, Yan L, Shames B. 1995. Evaluation of antibiotic therapies for eradication of Helicobacter hepaticus. Antimicrob Agents Chemother 39:1292-1294.
4. Foltz CJ, Fox JG, Yan L, Shames B. 1996. Evaluation of various oral antimicrobial formulations for eradication of Helicobacter hepaticus. Lab Anim Sci 46:193-197.
5. Fox JG, Dewhirst FE, Tully JG, Paster BJ, Yan L, Taylor NS, Collins MJ, Gorelick PL, Ward JM. 1994. Helicobacter hepaticus sp. nov., a microaerophilic bacterium isolated from livers and intestinal mucosal scrapings from mice. J Clin Microbiol 32:1238-1245.
6. Fox JG, Lee A. 1993. Gastric Helicobacter infection in animals: natural and experimental infections, p. 407-430. In Goodwin CS, Worsley BW (ed.), Helicobacter pylori: biology and clinical practice. CRC Press, Boca Raton, Fla.
7. Fox JG, Li X, Yan L, Cahill RJ, Hurley R, Lewis R, Murphy JC. 1996a. Chronic proliferative hepatitis in A/JCr mice associated with persistent Helicobacter hepaticus infection: a model of Helicobacter-induced carcinogenesis. Infect Immun 64:1548-1558.
8. Fox JG, Yan L, Dewhirst FE, Paster BJ, Shames B, Murphy JC, Hayward A, Belcher JC, Mendes EN. 1995. Helicobacter bilis sp. nov., a novel Helicobacter species isolated from bile, livers, and intestines of aged, inbred mice. J Clin Microbiol 33:445-454.
9. Fox JG, Yan L, Shames B, Campbell J, Murphy JC, Li X. 1996b. Persistent hepatitis and enterocolitis in germfree mice infected with Helicobacter hepaticus. Infect Immun 64:3673-3681.
10. Lee A, Phillips MW, O'Rourke JL, Paster BJ, Dewhirst FE, Fraser GJ, Fox JG, Sly LI, Romaniuk PJ, Kouprach S. 1992. Helicobacter muridarum sp. nov., a microaerophilic helical bacterium with a novel ultrastructure isolated from the intestinal mucosa of rodents. Int J Syst Bacteriol 42:27-36.
11. Mahler M, Bedigian HG, Burgett BL, Bates RJ, Hogan ME, Sundberg JP. Comparison of diagnostic methods for detection of Helicobacter species in laboratory mice. Lab Anim Sci (submitted).
12. Queiroz DMM, Contigli C, Coimbra RS, Nogueira AMMF, Mendes EN, Rocha GF, Moura SB. 1992. Spiral bacterium associated with gastric, ileal and caecal mucosa of mice. Lab Anim 26:288-294.
13. Riley LK, Franklin CL, Hook RR, Besch-Williford C. 1996. Identification of murine Helicobacters by PCR and Restriction Enzyme Analyses. J Clin Microbiol 34:942-946.
14. Russell R J, Haines DC, Anver MR, Battles JK, Gorelick PL, Blumenauer LL, Gonda MA, Ward JM. 1995. Use of antibiotics to prevent hepatitis and typhlitis in male scid mice spontaneously infected with Helicobacter hepaticus. Lab Anim Sci 45:373-378.
15. Schauer DB, Ghori N, Falkow S. 1993. Isolation and characterization of "Flexispira rappini" from laboratory mice. J Clin Microbiol 31:2709-2714.
16. Shames B, Fox JG, Dewhirst F, Yan L, Shen Z, Taylor NS. 1995. Identification of widespread Helicobacter hepaticus infection in feces in commercial mouse colonies by culture and PCR assay. J Clin Microbiol 33:2968-2972.
17. Taylor NS, Fox JG, Yan L. 1995. In-vitro hepatotoxic factor in Helicobacter hepaticus, H. pylori and other Helicobacter species. J Med Microbiol 42:48-52.
18. Ward JM, Anver MR, Haines DC, Benveniste RE. 1994a. Chronic active hepatitis in mice caused by Helicobacter hepaticus. Am J Pathol 145:959-968.
19. Ward JM, Anver MR, Haines DC, Melhorn JM, Gorelick PL, Yan L, Fox JG. 1996a. Inflammatory large bowel disease in immunodeficient mice naturally infected with Helicobacter hepaticus. Lab Anim Sci 46:15-20.
20. Ward JM, Fox JG, Anver MR, Haines DC, George CV, Collins MJ, Gorelick PL, Nagashima K, Gonda MA, Gilden RV, Tully JG, Russell RJ, Benveniste RE, Paster BJ, Dewhirst FE, Donovan JC, Anderson LM, Rice JM. 1994b. Chronic active hepatitis and associated liver tumors in mice caused by a persistent bacterial infection with a novel Helicobacter species. J Natl Cancer Inst 86:1222-1227.
21. Ward JM, Benveniste RE, Fox CH, Battles JK, Gonda MA, Tully JG. 1996b. Autoimmunity in chronic active Helicobacter hepatitis of mice. Serum antibodies and expression of heat shock protein 70 in liver. Am J Pathol 148:509-517.