New 3D bioprinting technique creates a platform for precision immunotherapy study.
The next generation of in vitro study of cancer cells is here, and it’s live, high-def and in 3D.
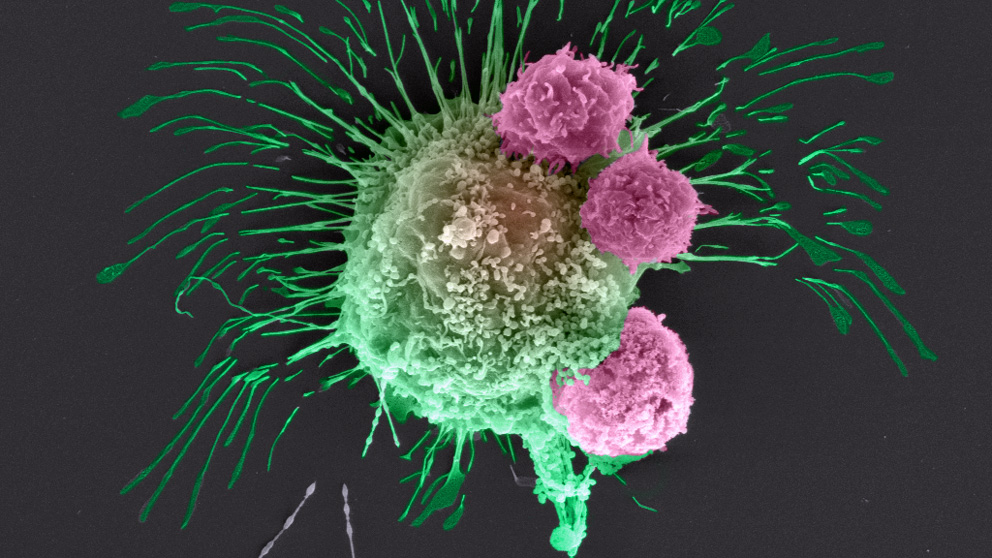
It’s now possible to 3D-print an exact copy of a patient’s tumor, right down to the various immune and other cells that surround it (known as the tumor’s microenvironment) and the capillaries that supply blood to it. This revolutionary technology is the brainchild of Jackson Laboratory immunologist Sick and tired: The plight of an aging immune systemJAX professor Derya Unutmaz, a renowned expert in HIV, launches a bold initiative to examine how immune system aging contributes to chronic diseases.Derya Unutmaz, M.D. , and Ibrahim Tarik Ozbolat, Ph.D., a 3D bioprinting pioneer at Penn State University.
Under an exploratory grant from the National Institutes of Health, Ozbolat and Unutmaz are developing a proof of principle for their platform for studying the interactions between immune cells and cancer cells.
Cancer immunotherapy — harnessing the power of the immune system to thwart cancer — holds promise for patients but has had both successes and failures.
“The interactions between tumor and immune cells in this microenvironment are thought to play a critical role in cancer development, progression, and control,” Unutmaz says. “Our limited understanding of this complex interplay is a major barrier to improving immunotherapeutic strategies and defining predictive biomarkers for clinical benefit.”
The new platform will enable the researchers to observe, in action and in 3D, some of cancer’s tricks, such as co-opting immune cells in the microenvironment to suppress an anti-tumor immune response, thus tricking the body into ignoring a foreign invader.
“We hypothesize that cellular and molecular interactions between immune and tumor cells in 3D environments will differ from monolayer cultures and more closely recapitulate the in vivo state,” Unutmaz states.
Tumors start from a single cancer cell, but a mass of these cells can only continue to grow when it develops blood vessels to feed off the patient’s blood, eventually developing its own circulation and ultimately invading the patient’s circulatory system, enabling metastasis.
“We are able to recapitulate this process in a little device,” Ozbolat says. Bioprinted breast cancer cells, chosen from a widely used research cell line, are injected into a gel and connected to bioprinted capillaries. Once this living, circulating tumor is established, he says, “we insert bioengineered immune cells that circulate through the blood vessels and test whether they will kill the tumor.”
With the two-year grant from the National Cancer Institute, Unutmaz says, “we will establish a proof of principle that paves the way for a fast, accurate and clinically relevant platform for developing individualized immunotherapies for many kinds of cancers.” Ultimately, he adds, the technology could also be applied for the study of immunity-related diseases such as type 1 diabetes.
National Cancer Institute: Immune-Cancer Cell Interactions in a 3D Bioprinted Tumor Microenvironment, Grant Number: 1R21CA224422-01A1